PHOTOXIDATION OF PHENOL IN AQUEOUS SOLUTION
Asmae MOKRINI, Driss OUSSI, Esther CHAMARRO, Santiago ESPLUGAS*.
Departamento de Ingeniería Quimica. Universidad de Barcelona.
C/ Marti i Franquès, 1. 08028 Barcelona.
phone 34-3 402 1288
fax 34-3 402 1291
email esplugas@angel.qui.ub.es
ABSTRACT
This paper presents the results of the oxidation of Phenol in aqueous solution in a semi-batch reactor. The advanced oxidation processes studied were UV, ozone, hydrogen peroxide and its combinations. The pH dependence and the influence of the initial concentration of hydrogen peroxide were studied to find the optimal conditions for a complete and fast oxidation of this organic compound.
Experimental results indicated that phenol is destroyed more rapidly by ozone at higher pH (9-12), while ozonation combined with hydrogen peroxide or/and UV is considerably faster at low pH (3-7).
KEYWORDS
Wastewater, phenol, ozone, UV, hydrogen peroxide, hydroxyl radical.
* To whom all correspondence must be addressed
INTRODUCTION:
The oxidation processes involving hydroxyl radical have shown their potential to destroy toxic organic compounds in waste-water. The main interesting characteristics of hydroxyl radical are its very high oxidation potential (bigger than ozone and only smaller than fluorine) and the possibility of its generation by different ways. This radical may be produced by combining ozone with ultraviolet light (Glaze et al.,1982; Mirat et al.,1987; Esplugas et al.,1994; Prado et al.,1994), ozone with hydrogen peroxide (Paillard et al.,1986; Trancart, 1990; Duguet et al.,1990), hydrogen peroxide with ultraviolet light, hydrogen peroxide with ferrous or ferric ion (David et al.,1991; Joseph J.,1992) and by photocatalysis, which uses a semi conductor in combination with visible and UV radiation and molecular oxygen (Weir et al.,1990; Brezova et al.,1991).
Ozonation is a simultaneous mass-transfer/reaction-kinetics phenomenon. Kinetic modeling of the ozonation of phenol in water has been studied by Roth et al. (1982), using a semi-empirical model which incorporate the mass transfer, resulting from the diffusional resistance of ozone, and kinetics encountered during ozonation with operating parameters for scale-up for wastewater treatment. The combination of ozone and hydrogen peroxide is used essentially for the contaminants which oxidation is difficult and consumes large amounts of oxidant. Because of the high cost of ozone generation, this combination makes the process economically feasible. The photolytic ozonation (uv/ozone) was also used for the oxidation of phenolic compounds; Gurol et al. (1987) studied the photolytic ozonation of mixtures of phenols, TOC removal by UV light alone was negligible, by ozone 30% has been removed and more than 95% by UV/ozone.
In order to compare the different advanced oxidation processes phenol has been chosen because it is a very simple organic compound, easily soluble in water at different condition of acidity. In addition phenol constitutes one of the main pollutants to be removed from wastewater.
EXPERIMENTAL
All experiments were performed in the experimental installation shown in figure 1. The reactor is tubular with a spherical vessel with 4.54 L capacity upper. The design of this reactor possibilities the cocurent circulation of gas (oxygen and generated ozone) and liquid (aqueous solutions of the compounds studied) which are mixed with a diffuser valve in the bottom of the reaction tube.
Oxygen gas was supplied in standard cylindrical tanks and fed to an ozone generator model TDZ 11-20 TODOZONO. The saturation concentration of ozone was determined to be 1.5-2 mg/L depending on the flow rate of gas through the system. The reactor is surrounded by four low mercury lamp (15 Watts each lamp) which emit radiation at 253.7 nm.
For every experiment, the reactor was filled with 2500 cm3 of aqueous solutions of analytical grade phenol (from PROBUS), buffered with 0.01M Na2HPO4/0.01M KH2PO4 to adjust the pH at 6.9. A 0.05M borax buffer was used to adjust the pH of phenol solutions at 9.3.
The samples were taken at appropriate time intervals and analyzed immediately by high pressure liquid chromatography (HPLC) using a Waters chromatograph. The analyses were made in reverse phase with an ODS2 SPHERISORB column eluting the injected sample (20 m L) with a mixture of acetonitril-water (50:50 v/v) in the case of phenol, with a flow rate of 1 cm3.min-1.
.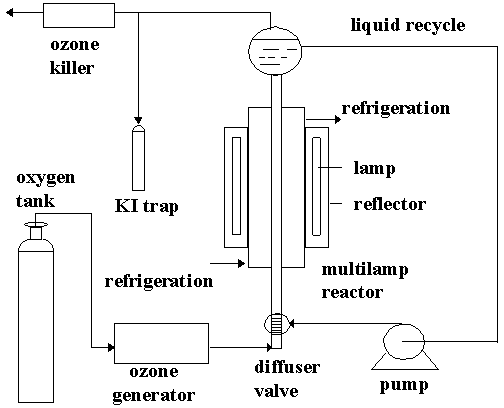
Figure 1. Experimental installation.
For all experiments done with ozone, the ozone gas excess was passed out through the top of the reactor into a bottle containing 2% KI solution for the later iodometric determination of ozone production (Birdsall, 1952). The concentration of residual dissolved ozone was followed by the Indigo procedure (Hoigné and Bader, 1983). A spectrophotometer SHIMADZU model UV-1203 was used to measure the change in absorbance of the indigo.
In addition, samples were also analyzed to determine the total organic carbon (TOC), using a Dohrman DC-190 high level TOC analyzer.
The operating conditions in the experimental series were:
ozone production = 0.2 - 0.3 g.h-1
radiation intensity = 7.22 µeinstein.s-1
gas flow rate = 60 ± 4 L.h-1
liquid recirculation rate = 100 ± 5 L.h-1
reaction volume = 2.5 L
temperature = 18 - 25 ºC
RESULTS AND DISCUSSION
Figures 2 shows the rate of disappearance of phenol at no buffered acid pH (4.9) using different AOP. It can be observed that the combination of UV/Ozone/Hydrogen Peroxide leads to a better degradation of the phenol. In this unbuffered experiments, the pH dropped (3.2 ) due to the formation of acidic intermediates and products.
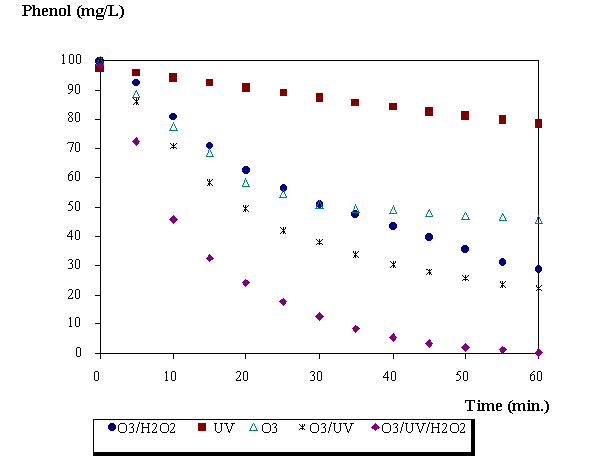
Figure 2. Profiles of phenol with different AOP. pH=4.9, [H2O2] 0=3.19.10-3 mol.L-1
The photolysis with 253.7 nm radiation practically does not degrade phenol. But the combination UV/ozone gave better results than the using only ozone. In the degradation of phenol using ozone there are two main reactions: the direct reaction of ozone with phenol and its products and the reaction of the hydroxyl radical generated. Some intermediate products as benzoquinone, catechol and hydroquinone were detected during the degradation of phenol (figure 3).
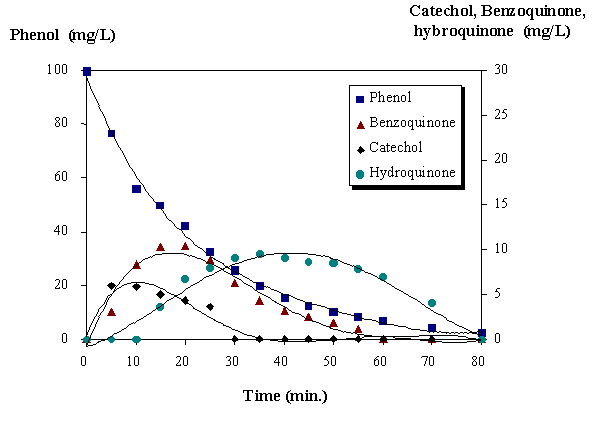
Figure 3. Profiles of phenol, benzoquinone, catechol and hydroquinone.
AOP: O3/UV/H2O2. pH=4.9, [H2O2] 0=1.59.10-1 mol.L-1.
Additionally, it must be taken into account the low rate of ozone decomposition at acid pH (Gurol and Singer, 1982) and the predominant direct reaction with molecular ozone. Ozone in the exit gas appeared few minutes after the beginning of all experiments with ozone (figure 4).
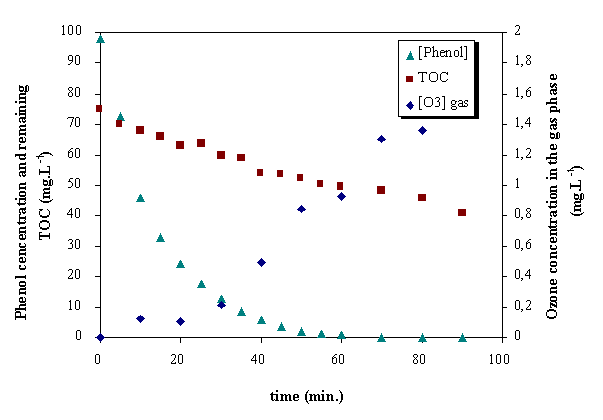
Figure 4. Profiles of phenol, TOC remaining and ozone in gas phase.
O3/UV/H2O2 , pH=4.9, [H2O2] 0=6.37.10-2 mol.L
The influence of initial hydrogen peroxide concentration have been studied in the case of phenol, several experiments were carried out with different initial concentrations of hydrogen peroxide. It was observed that the oxidation rate of phenol first increases when hydrogen peroxide concentration increases, the opposite effect was observed at high concentrations. 63.7 mmol/L was found to be the optimal concentration and it corresponds to the removal of all phenol and 40% TOC after 70 min. reaction time.
The pH is certainly the most important variable in advanced oxidation processes. In the experiments performed with the system UV/ozone, as it is shown in figure 5, when the pH increases the degradation rate also increases. The pH influence has been studied for the other oxidation processes, with the pH range used (4.8-5.2 for acidic pH, 6.8-7.1 for neutral pH and 9-9.5 for basic pH) the experimental results obtained show that ozonation, UV/ozono and ozone/hydrogen peroxide are faster at basic pH, while photolysis and UV/ozone/ hydrogen peroxide have a faster reaction rate at low pH values.
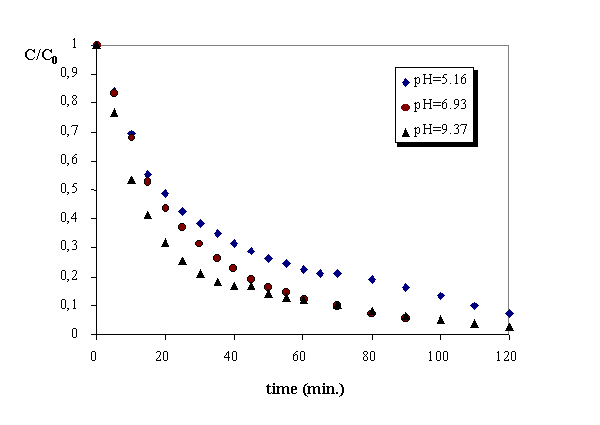
Figure 5. Influence of pH in the uv/ozone process.
More interesting is the influence of the presence of radical scavengers in the aqueous medium in the degradation rate. As it is shown in figure 6 the presence of t-butanol and the anion hydrocarbonate decreases drastically the degradation rate. After one houre reaction time the conversion is nearly 100% in the case of ozonation and only 75% adding radical scavengers. This result confirms that the free radical pathway is significant at higher pH values.
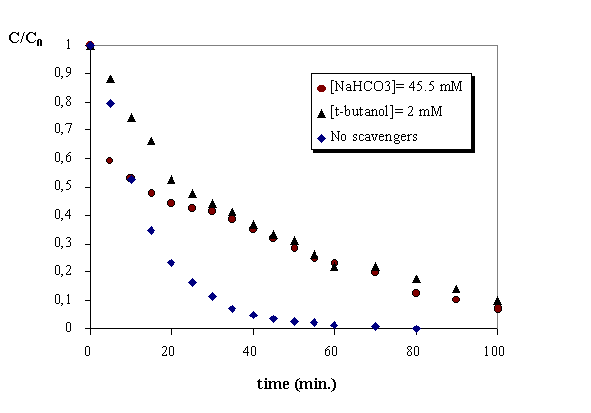
Figure 6. Ozonation at basic pH. Influence of the presence of scavengers.
CONCLUSIONS
All the Advanced Oxidation Processes used in this work leads to the removal of the compounds studied, but with different degradation rates. It is possible to mineralize the organic compounds.
The pH is an important variable to take into account. At acid pH, the direct attack by molecular ozone is predominant and any source of radicals (H2O2 or/and UV) improve the efficiency of ozonation, while at neutral and basic pH there is a major contribution of the free-radical pathway and the addition of hydrogen peroxide and/or UV can promote or inhibit the reaction of ozonation.
The presence of scavengers of hydroxyl radicals as t-butanol and sodium bicarbonate decreases the rate of phenol oxidation at higher pH values..
ACKNOWLEDGMENT
The support of DGICYT (project AMB96-09) is gratefully acknowledged.
REFERENCES
Birdsall, C. M. et al. (1952). Iodometric determination of ozone. Anal. Chem. 24(4), 662-664.
Brezova V., Ceppan M.,Brandstetova E.,Breza M., Lapcik L.(1991) Photocatalytic hydroxylation of benzoic acid in aqueous titanium dioxide suspension . J.Photobiol. 59 (3), 385-391.
David L., Sedlak and Andres W.Andren (1991) Oxidation of chlorobenzene with Fenton’s Reagent. Environ.Sci.Technol. 25, 777-782.
Dugnet J.P., Anselme C., Mazonnie P. and Mallevialle J. (1990) Application of combined ozone 1-292. -hydrogen peroxide for the removal of aromatic compounds from a ground water. Ozone Sci.Engng. 12, 28
Esplugas S., Yue P.L. and Perez M.I. (1994) Degradation of 4-chlorophenol by photolytic oxidation. Wat.Res. 28, 1323-1328.
Glaze W.H., Peyton G.R., Lins S., Huang F.Y. and Burleson J.L. (1982) Destruction of pollutants in water with ozone in combination with ultraviolet radiation. Environ.Sci.Technol. 16, 454-461.
Gurol, M. D. and Singer, P. C. (1982). Kinetics of ozone decomposition: a dynamic approach. Environ. Sci. Technol., 16(7), 377-383.
Gurol, M. D. et al. (1984). Kinetic behavior of ozone in aqueous solutions of substituted phenols. Ind. Eng. Chem. Fundam., 23(1), 54-60.
Hoigné, J. and Bader, H. (1983). Rate constants of reactions of ozone with organic and inorganic compounds in water. Wat. Res., 17, 173-183.
Joseph J. Pignatello (1992) Dark and photoassisted Fe3+-catalysed degradation of chlorophenoxy herbicides by hydrogen peroxide. Environ.Sci.Technol. 26, 944-951.
Mirat D. Gurol and Robert Vatistas. (1987) Oxidation of phenolic compounds by ozone and ozone-UV radiation: A comparative study. Wat.Res. 21, 895-900.
Paillard H., Brunet R. and Dore M. (1988) Conditions optimales d’application du systeme oxydant ozone-peroxide d’hydrogene. Wat.Res. 22, 91-103.
Prado J., Arantegui J.,Chamarro E. and Esplugas S. (1994) Degradation of 2,4-D by ozone and light. Ozone Sci.Engn. 16, 235-245.
Roth, J. A. et al. (1982). Kinetic modeling of ozonation of phenol in water. Journal WPCF., 54(1), 135-138.
Trancart J.L. (1990) Treatment of triazines in water-works: efficiency of O3/H2O2 coupling. Ozone New 18, 7-8.
Weir B.A. and Sundstrom D.W. (1993) Destruction of trichloroethylene by UV light catalysed oxidation with hydrogen peroxide. Chemosphere. Vol 27, 1279-1291.