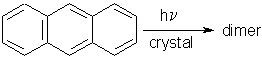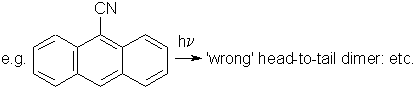SNOM (scanning near-field optical microscopy),
a genuine photochemical technique
Gerd Kaupp, Andreas Herrmann, Michael Haak,University of Oldenburg, FB9-OC I, Postfach 2503, D-26111 Oldenburg, Germany;
fax: +49 (0)441-798-3409
http://kaupp.chemie.uni-oldenburg.de/
Introduction, Physical Background:
SNOM (Scanning near-field optical microscopy; sometimes also called NSOM) represents a new field of research and a new photochemical technique. We have both a new ultramicroscopy technique and a new light source: it is a genuine photochemical device. Sooner or later it will be in textbooks and in modern lectures on photochemistry. The major prospects are shown in Figure 1. We deal with Rayleigh, Raman, absorption, fluorescence, polarisation phenomena.
|
(SNOM)
|
Figure 1: Prospects of SNOM
Some background is required. We have eight different types of SNOM and light-tunnelling as depicted in Figure 2.
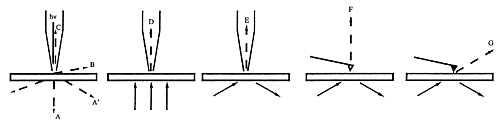
Figure 2: Basic types of SNOM
The techniques D,E,F,G require transparent samples and no commercial instruments are available. The techniques A/A', B and C are available with commercial instruments using horizontally vibrating tips under shear-force vibration-damping control [1]. However, the still most popular brands work with metal coated tips in the modes A, A', and B with extreme drawbacks that are only avoided in mode C with instruments that use uncoated tapered fiber tips. The difference is immediately seen in Figure 3.
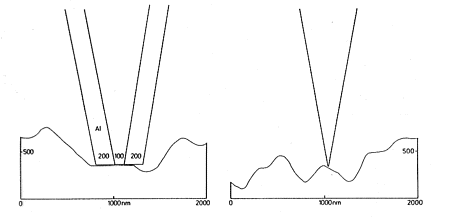
Figure 3: Coated and uncoated tips on rough surfaces
Metal coated tips are very blunt. They cannot follow topography but raze it off, when scanned in distances of less than 10 nm over the surface. Furthermore, they become very hot (> 100 °C up to 400 °C at apertures of 100 nm or below) and heat or melt the surface at below 10 nm distance. Only sharp tips without metal coating can follow topography and stay cold when illuminated. The optical resolution is only limited by the tip-radius (15 nm at present). These simple facts should be considered prior to the purchase of an instrument. It does not help much to perform constant height instead of constant distance measurements as in Figure 4 (b). Distance modulations rather than optical properties are measured in addition to the recording of interference fringes that are formed by reflections between the metal surface and the sample surface.
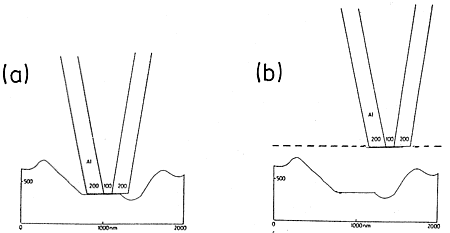
Figure 4: Impossible constant distance (< 10 nm) and constant height mode with metal-coated tips on rough surfaces
The technique with the uncoated tips avoids all of these errors due to the sudden increase of reflectivity back to the fiber as the vibrating tip gets close to the surface in the shear-force region at a damping of the amplitude of about 50% (below 10 nm, usually around 5 nm distance). That effect is now called Courjon-effect. It is shown for a tip with 15 nm end-radius approaching a uniform organic polymer surface in Figure 5 [2].
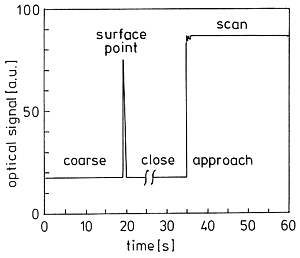
Figure 5: Approach record of a sharp uncoated tip
Starting coarse approach at 0.5 - 1 mm distance (t = 0) with the instrument in Figure 6, the increase of back reflection when the tip experiences the vibration damping provides the trigger signal for the instrument to stop approach and put the tip backward at a preset adjust position usually 1 µm above the surface from here it awaits the order by the operator to start careful close approach to the preselected damping level, where the reflectivity is largely increased (here 4.8 fold on the chosen polymer). It is only the enhanced close near-field intensity variation that gives rise to chemical SNOM contrast. The horizontal base-line proves the absence of interference effects. Thus, we do not get topographic artefacts when following the surface in constant distance (more precisely: at constant vibration damping by "shear force"). Tips that do not give the enhancement are unsuitable for SNOM measurements. That principle is realized in the commercial instrument RASTERSCOPE 4000 SNOM of DME, as shown in Figure 6.
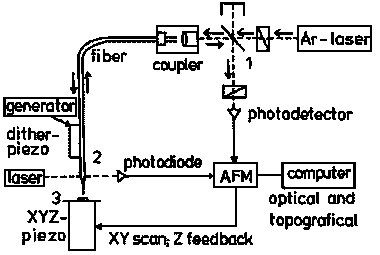
|
Figure 6: Setup of a reflection-back-to-the-fiber SNOM; 1: beam-splitter and crossed polarizers; 2: shear-force damping control; 3: sample table. |
First of all, this is a non-contact AFM (Atomic Force Microscope) under atomic shear-force control. It keeps the vibrating tip at a constant "shear-force" distance (any slight variations of the shear-force response belong to the chemical properties) with the sample via damping of its amplitude. By doing so, it provides an AFM image simultaneously with the SNOM, if the optical part is switched on. The Argon-laser (or any other high quality laser) lights the first polarizer (set at max. throughput) of the crossed polarizer system designed to minimize straylight by passing only light from the back-reflection into the fiber that is depolarized. The light is coupled into an optical fiber that is tapered and ends with a tip radius of 15 - 20 nm. The light coming down interacts in the close near-field and is efficiently reflected only in the tip radius region (see Figure 5). It is coupled out by the beam splitter and detected and processed to a SNOM image that is totally decoupled from the simultaneous topographical image. The scanning over the surface is provided by the XYZ piezo element using efficient Z-feedback by the shadowing control of a small auxiliary laser and photodiode. That setup can also be run under shear-force control without scanning for local spectroscopy and local photochemistry at preselected spots.
Applications:
A. Chemical Contrast:
It is of major importance to evaluate the local chemical nature of the surface
features that form in solid-state photochemistry or solid-state reactions
with gases and between solids.
The versatile SNOM technique that can handle roughness without introducing
artefacts is the first that is able to record chemical contrast of surface
features, but only if these differ chemically (or physically) from their
environment (chemical uniform rough surfaces do not give SNOM-contrast).
We have published numerous examples [1, 3].
1. The autooxidation of solid 2-mercaptobenzothiazole (benzothiazoline-2-thione)
on its (001) face is crystallographically hindered, because the functional
groups are shielded by the packing.
The packing [4] is shown in Figure 7 facing (001).
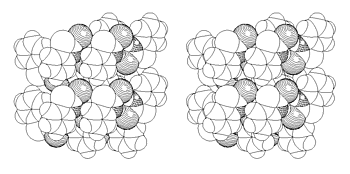
In view of the steric hindrance on the surface, only an island mechanism is possible around defect sites that serve as nucleation centers. Figure 8a shows the AFM image of such islands with up to 500 nm height. These grew out of the flat surface. We had to prove that the islands are indeed chemically different from the solid "sea" around them. SNOM in Figure 8b gives the answer. Only the islands give dark optical contrast. The disulfide is less reflective than the unaffected starting material, probably because it is less polar than the starting material. The theory of near-field reflectivity is still subject to theoretical evaluation. Our results provide the empirical basis for that endeavour. There is also an influence of specific shear-force response that belongs to the chemical contrast, of course.
 a: AFM, Z-scale 500 nm |
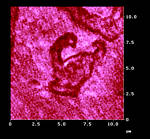 b: simultaneous SNOM |
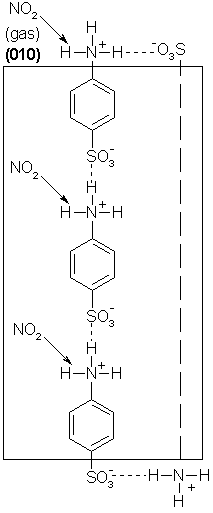
2. Solid state diazotation is a valuable technique [5].
Figure 11 shows that the reactive 9,10-positions are inaccessibly hidden under the (001) face.
Thus, the reaction has to develop around defect sites.
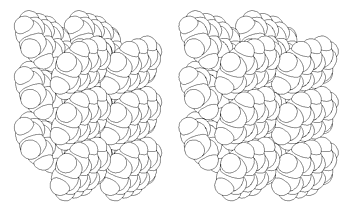
Figure 11: Space filling stereoscopic view of the crystal packing showing two layers of anthracene under (001)
Indeed, the AFM in Figure 12a displays beautiful islands of up to 200 nm height. However, AFM alone cannot prove a chemical difference between the reacted and unreacted parts of the surface. Only the optical contrast in SNOM (Figure 12b) proves that the islands are chemically different. As they formed from a flat surface it is clear that they consist of the product that is identified as 9,10-dibromoanthracene after completion of preparative runs. Here the product reflects less intensely in the close near-field than the starting material. What we see in Figure 12b is genuine chemical contrast.
 a: AFM, Z-scale 200 nm |
 b: simultaneous SNOM |
B. Mechanistic Questions
The solid state photodimerization of a-trans-cinnamic acid is historically important.
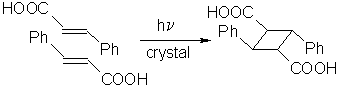
It was used to initiate a claim of minimum atomic and molecular movements in solid-state reactions [7],
which is still found as "Schmidt“s topochemistry" in almost all textbooks of photochemistry.
However, AFM investigation showed that this (Ar, N2, air, vacuum; irrespective of wavelength)
and all other studied non-topotactic solid-state reactions require long-range molecular movements.
That fact is evident from the surface features that build up on the surface in two steps
(1. phase rebuilding and 2. phase transformation) prior to crystal disintegration.
Thus, we do not have minimal movement but the requirement for long-range molecular
movements that are guided by the crystal packing which relates to the shape of the characteristic features.
That change in paradigm (a claim that long-wavelength irradiation gives a topotactic reaction of
a-trans-cinnamic acid has been definitely disproven:
the reported x-ray analysis is faulty [1, 8, 9]
and it is not possible to avoid crystal disintegration above 20% conversion even though we could shift
the disintegration point up to 30% conversion at extremely long-wavelength, still feature-forming irradiation) requires sound evidence from different techniques [1].
It remained to show chemical uniformity on the photo-reacting surface of a-trans-cinnamic acid.
Solid a-trans-cinnamic acid shows a long wavelength ct-band and an onset that starts in the visible
(Figure 13). Irradiation at all wavelengths that are absorbed by the solid
leads to very characteristic surface features and disintegration at low overall conversions,
precluding single crystal x-ray analysis up to 100 % conversion.
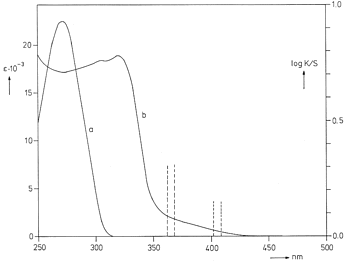 Figure 13: a) UV spectrum of a-trans-cinnamic acid in methanol; b) diffuse reflection spectrum of a-trans-cinnamic acid powder |
Figure 14 shows the development of the beautiful features on (010) of a-trans-cinnamic acid at 365 nm irradiation under various conditions (similar features also at 405 nm in air, under N2 or in a vacuum) in the AFM and Figure 15 shows the features at extremely long-wavelength irradiation (6 months with filtered daylight) at 30% conversion when starting the crystal disintegration starts [8].
 |
 |
 |
 |
 |
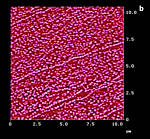
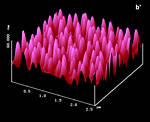
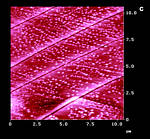
Figure 14: AFM surfaces of a-trans-cinnamic acid; a: fresh, Z-Scale 10 nm; b/b': after 45 min 365 nm irradiation; c: 45 min hv, 40 min delay, 45 min hv; d: new sample after 150 min uninterrupted hv; Z-scale in b,c,d 100 nm; light intensity: 6.0 mW/cm2 the ridges and fissures are at 40° to the long crystal c-axis in all cases |
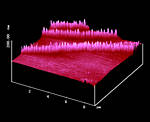
Figure 15: AFM surface (010) of an unbroken part of a-trans-cinnamic acid
after 6 months daylight through Pyrex close to a North-side window in Oldenburg, Germany
All features relate to the crystal packing. In none of the reaction stages of Figures 14 and 15 was it possible to detect a SNOM contrast. That is indicated in Figure 16 for a still further reacted crystal of a-trans-cinnamic acid and shows that we have chemical uniformity at any stage of the reaction on the surface but not accumulation of the dimer a truxillic acid at the features. The slight features in the SNOM at the very steep and high (1.5 µm) edges of the AFM features are an indication for a steepness contrast that does not significantly exceed the uncorrelated roughness.
 a: AFM, Z-scale 1,5 µm |
 b: simultaneous SNOM (missing significant contrast) |
Similarly, the mechanism of anthracene photodimerization, that had been revised with AFM, has been completed using SNOM.
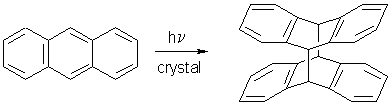
There was a feeling in photochemistry textbooks and literature that the "topochemically forbidden" photodimerization of anthracene (d = 6.038 Å) be a defect site reaction with the assumption that the electronic energy migrates to the defect-sites where the reaction "might occur". Figure 17 shows the AFM results on (001) and on (110) of anthracene [6].
 |
 |
|---|---|
 |
 |
|
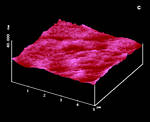
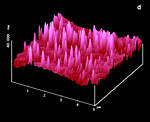
Figure 17: AFM surfaces of anthracene; a: fresh on (001) with molecular steps b: after 2 min irradiation; c: fresh on (110); d: after 1 min irradiation; |
In the defect-site theory the features should be the product and the flat parts unreacted. However, the long-range movements with face-specific features disprove that assumption: SNOM did not give a contrast at these stages or at more advanced stages of the reaction. Thus, we have chemical uniformity on the surface despite face-specific feature formation. The dimer forms where the light is absorbed and molecules move along the easiest paths in the lattice. The sketch in Figure 18 explains why the molecules on (001) exit a the molecular steps. On (110) the packing situation is similar to the vertical sides of the sketch and therefore different features (vulcanoes) ensue [10].
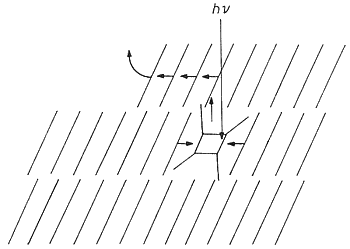
Figure 18: Scheme for one orientation of the steeply layered anthracene molecules under (001) and for the molecular movements after photodimerization in the crystal bulk.
Thus, the enormous theoretical effort to predict electronic energy migration does not help for untenable minimal movement claims. It has been demonstrated that anthracene molecules have an easy way to form the dimer despite much larger distance than the alleged 4.2 Å [1].
The trouble with topochemistry in the restrictive sense of Schmidt since 1964 [7] may be summarized with just a few out of many long-known "not fitting" reaction examples in Figure 19. It also indicates how AFM evidence tells what is really going on in solid-state photochemistry.
| New Mechanism: AFM evidence tells: not minimal movements from <4.2 to 1.5 Å, but molecules must move from far distances and rebuild the phase (a paradigmatic change!) | ||||||||
|
||||||||
| Conclusion for anthracene: AFM shows face-specific surface features, SNOM indicates chemical uniformity on the surface, thus, this is not a defect-site reaction.
Similarly, all other drawbacks of topochemistry just vanish (1991) | ||||||||
Figure 19: Some of the drawbacks of Schmidt“s topochemistry and explanations on the experimental evidence of long-range movements that are strictly guided by the molecular packing.
Long-range movements have to find a reasonable easy way in the crystal lattice. In unfavourable cases the packing may even impede very short movements. The limiting-distance issue was detracting from crystal packing considerations. Anthracene does photodimerize and that is one of the earliest photoreactions studied, whereas stilbene does not photodimerize in the solid state. The 9-cyanoanthracene issue has been clarified [1, 6]: The packing allows only for the head-to-head dimer to be formed. However, once formed with phase rebuilding it is thermolabile and reverts to the starting compound with still further phase rebuilding. Further light absorption finds its way to form the thermally stable head-to-tail dimer.
C. Photolithography
9-Chloroanthracene may be photodimerized at 488 nm. A poor uncoated tip was placed
at three preselected spots under shear-force distance control and irradiated in Figure 20.

Figure 20: Photolithography on a crystal surface of 9-chloroanthracene with a poor illuminated tip at three preselected spots.
Large features with up to 2 µm diameter were formed. These are too large for data storage but such photolithography is a rapid technique to judge and eliminate unsuitable tips. Furthermore, we see, that large areas are illuminated by uncoated tips [11].
Unsurpassed photolithography has been obtained with good, far-field apertured tips and thermally insulated thioindigoid dyes without H-bridges [12].
Such solid dye surfaces formed very sharp features by local radiationless decay with heat formation rather than photoreaction. The local heat leads to local melting (m.p. 172 °C) with feature formation. Figure 21 exhibits a rough starting surface that was sluggishly crystallized in order to produce some landmarks.
 |
 |
|---|---|
 |
 |
|
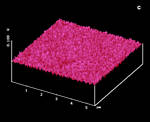
Figure 21: Photolithography on the thioindigoid dye surface; a: AFM, fresh; b: AFM after 10 s irradiation at two preselected spots c: simultaneous SNOM image; d: AFM after extreme local overirradiation, new sample |
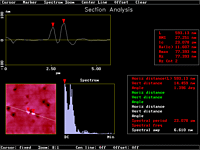
Figure 22: Cross section analysis of Figure 21b |
The tip was put at the preselected left spot under shear-force control and illuminated at 1 µW output (488 nm) for 10 s in Figure 21b. Then at the close second spot in its center. What we got is a promising technique for ultra high data storage. The two features are 600 nm apart (Figure 22). The dynamics of the process are very high. Figure 21d is the result of an unsuitable tip that was illuminated for 45 s at 80 µW under shear-force control on a different crystal of the same dye. It still shows sharp contoures despite extreme overirradiation. SNOM secures, that no chemical change occurs because we do not obtain any chemical contrast in Figure 21c.
The mechanism of the cone formation was secured by partial melting of the crystal surface with an IR-heater and recooling under microscopic control. Figure 23 shows that cones formed indeed on that surface, probably due to surface tension.
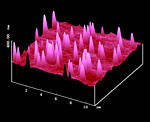
Figure 23: AFM after cone forming partial surface melting of the thioindigoid dye with an IR-heater
Data storage at that level should be augmented by the usual array and replica techniques. The reading by AFM is a feasible option. The performance of that lithography without development steps is best judged by the present state of the art in high density data storage.
|
High density information storage
1995: US National Medal of Technology to IBM |
|
"Ultrahigh" density information storage on organic crystals (... replica etc.)
1997: very first experiment: 90 MB/cm2 (calc.) These are more easily read than atomic scale features !
Replica will be stable enough in the long term |
Figure 24: Comparison of state of the art data storage and prospects of SNOM photolithography on organic crystals
Figure 24 gives the award-winning prospects on top and the prospects of our new technique on bottom, a 35.000 fold increase that looks for support to be transformed into a technical product.
E. Local Spectroscopy
The ultimate goal of chemical SNOM analysis is local fluorescence and Raman spectroscopy.
While we showed with several fluorescent textile dyes that the collected near-field
fluorescence can be separated out by edge filters, it is a matter of improving and
adjusting spectrometers to the SNOM in a way that the locally collected light can
be spectrally analyzed. The near-field effect is clearly demonstrated, the validity
of our measurements independently secured (correlation of chemically based topography
with the SNOM contrast is, of course, strictly necessary; deviations from the obvious
relation are a clear indication of artefacts in the measurement).
In preliminary test measurements with an optoacoustic spectrometer
of IFU in Floeta, Germany, we obtained a much better resolved local Raman spectrum with the
519.7 cm-1 Si-line (half width: 4.8 cm-1) under shear-force distance control in 10 minutes by
subtraction of the Raman response of the fiber (Figure 25).
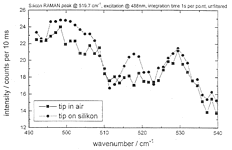
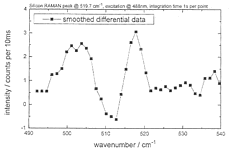
Figure 25: Local Raman spectra of a quartz-glass fiber tip in air, on silicon, and smoothened difference spectrum
It was only necessary to collect 100 spectra (1 s integration time per point) and we also got the Raman emission around 500 cm-1 of the SiO2 cover of Si. The superiority of our technique over the recent most advanced Raman spectroscopy with aperture SNOM [13] is evident, if the reported collection time (9.1 h !) at lower spatial (150x150 nm>SUP>2 area scan) and spectral resolution (7.5 cm-1) and the absence of the 500 cm-1 SiO2-band is considered. We hope to get granted the spectrometer whose sensitivity is being increased further for Raman-spectroscopy of organic local features at high spatial and spectroscopic resolution on rough surfaces.
Acknowledgements
We thank the Fonds der Chemischen Industrie and BASF AG, Ludwigshafen for financial support.
The Deutsche Forschungsgemeinschaft provided a travel grant and 2 years BAT IIa/2 position
in a parallel project that helped to install the privately purchased SNOM and periphery.
G. K. thanks his wife Maria Kaupp for her consent and support to the private purchases.
Literature
[1] G. Kaupp, Supermicroscopy in Supramolecular Chemistry: AFM, SNOM, and SXM, in Comprehensive Supramolecular Chemistry, Vol. 8, 381-423 + 21 color plates, ed. J. E. D. Davies, Elsevier, Oxford, 1996.[2] G. Kaupp, A. Herrmann, SNOM by Near-Field Reflectance Enhancement: A Versatile and Valid Technique, J. Phys. Org. Chem. 1998, 11, in press.
[3] G. Kaupp, A. Herrmann, Chemical Contrast in Scanning Near-Field Optical Microscopy, J. Phys. Org. Chem.1997, 10, 675-679.
[4] J. P. Chesiek, J. Donohue, Acta Cryst. 1971, 27, 1441.
[5] G. Kaupp, A. Herrmann, Abfallfreie Gas/Festkörper Diazotierung mit Stickstoffdioxid und quantitative Triazensynthese ohne Flüssigphase, J. prakt. Chem./Chem.-Ztg., 1997, 339, 256-260.
[6] G. Kaupp, M. Plagmann: Atomic Force Microscopy and Solid State Photolyses: Phase Rebuilding, J. Photochem. Photobiol. A: Chem. 1994, 80, 399-407.
[7] M. D. Cohen, G. M. J. Schmidt, F. I. Sonntag, J. Chem. Soc. 1964, 2000.
[8] G. Kaupp, M. Haak, Phase Rebuilding of a-Cinnamic Acid in Tail Irradiations, Mol. Cryst. Liq. Cryst. 1998, 313, 193-198.
[9] G. Kaupp, M. Haak, A. Herrmann, Atomic force and near-field optical microscopy in organic photochemistry, XVIIIth Int. Conf. on Photochemistry, Warsaw, 8. 8. 97; see errors in the packing diagram as calculated from the reported data
[10] G. Kaupp, Supermikroskopie in der Chemie, Chemie in unserer Zeit 1997, 31, 129-139; english translation.
[11] G. Kaupp, J. Schmeyers, M. Haak, T. Marquardt, A. Herrmann, AFM in Organic Solid State Reactions, Mol. Cryst. Liq. Cryst. 1996, 276, 315-337.
[12] G. Kaupp, A. Herrmann, Positive submicron lithography using uncoated or far-field apertured SNOM tips on organic crystals, Ultramicroscopy 1998, 71, 383-388.
[13] S. Webster, D. N. Batchelder, D. A. Smith, Submicron resolution measurement of stress in silicon by near-field Raman spectroscopy, Appl. Phys. Lett. 1998, 72, 1478.