 |
TYPE II PHOTOSENSITIZATION BY NSAID IN MICROHETEROGENEOUS SYSTEMS |
| Luis A. Martínez, André M. Braun and Esther Oliveros | |
| Lehrstuhl für Umweltmesstechnik, Engler-Bunte-Institut, Universität Karlsruhe, D-76128 Karlsruhe, Germany |
1.- INTRODUCTION
Microheterogeneous reaction systems may induce considerable modifications in rates, yields and products of photochemical reactions [1]. This is mainly due to their ability to solubilize the reactants in different microdomains, leading to polarity, concentration and aggregation effects. Photobiological reactions always occur in a microheterogeneous cellular environment which may highly influence their course. Therefore, model experiments in homogeneous solution might not lead to conclusive results when the photoreactivity of molecules of biological interest is to be investigated. Micellar media and microemulsions may provide simple models for mimicking cellular environments [2].
We have recently shown [3] that non-steroidal anti-inflammatory drugs derived from 2-arylpropionic acid (NSAID, Figure 1) are efficient singlet oxygen (1O2) sensitizers in microheterogeneous systems (four-component anionic microemulsions), the quantum yields of 1O2 production (FD) being higher than those found in (homogeneous) solution. There is also evidence that the photoproducts derived from the drugs behave as potent 1O2 sensitizers.
In this work, we have focused
our attention on the parameters which may influence the overall 1O2
sensitization process in organized media, such as: different types of surfactants
(anionic and cationic), pH values and the ability of some NSAID to quench
(either physically or chemically) 1O2.
![]()
2.- EXPERIMENTAL DETAILS
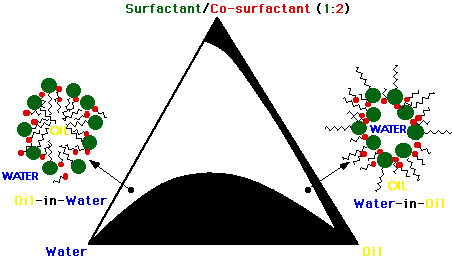
2.1 Microheterogeneous systems
All oil-in-water (O/W) and
water-in-oil (W/O) microemulsions (prepared using triply distilled water
or phosphate buffer 0.02 M, pH 7.2) contained the same weight of surfactant
(SDS, CTAC) and co-surfactant (1-butanol) in a ratio of 1:2. O/W microemulsions
were prepared using D2O in order to obtain luminescence signals
of appropriate intensity. The pseudo-ternary phase diagram is shown Figure
2. Micellar solutions contained up to 0.2 M of the surfactant in D2O.
No micellar solutions could be prepared, at room temperature, using phosphate
buffer instead of water.
2.2 Luminescence measurements
We have monitored the weak
1O2 luminescence at 1270 nm to measure its production
upon continuous irradiation of the sensitizers [4].
Briefly, the emission is detected as an electrical signal which is directly
proportional to the quantum yield of 1O2 luminescence
(Fe).
The quantum yield of 1O2 production (FD)
may be calculated from Equation 1 if some conditions are fulfilled: same
medium, matched absorbances at l
of excitation, quenching by the reference negligible when compared with
non-radiative 1O2 deactivation by the solvent (kd).
When kd and the rate constant of 1O2 quenching
by the drug under study (ktsens) are not known in
a given medium, an apparent 1O2 quantum yield (FDapp)
may be estimated from Equation 2 [5].
Equation 1. S and SR are the electrical signals measured for the drug and the reference sensitizer, while Po and PoR represent the corresponding incident photon rates at l of irradiation.
![]()
3.- RESULTS AND DISCUSSION
3.1 Reference sensitizers
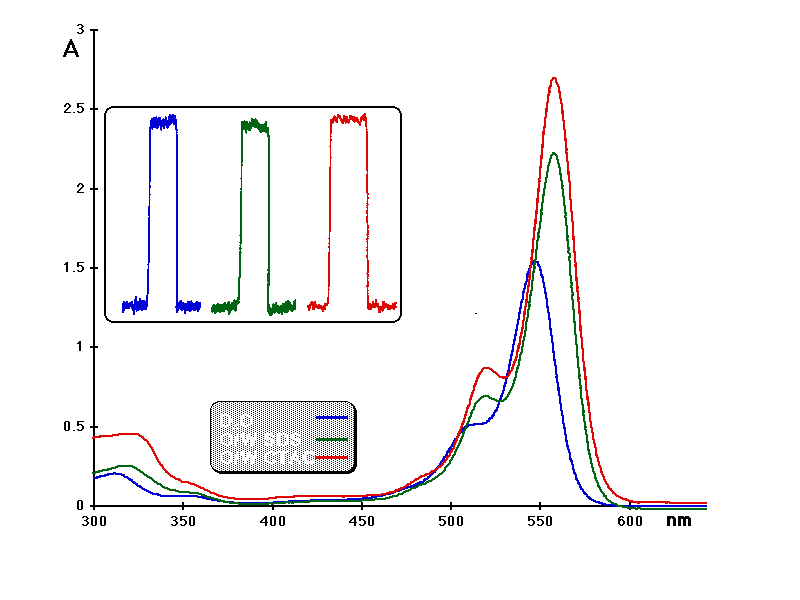
The well-known 1O2 sensitizers rose bengal (RB) and phenalenone (PN) were selected as references (values of FD of 0.76 and 0.98, respectively, were used). Absorption maxima of RB in microheterogeneous systems were red shifted compared to water. According to the spectral shifts (Figure 3), RB is exposed to a butanol-like environment when dissolved in microheterogeneous systems. It has been shown that the values of FD of RB are not modified in water and SDS micelles (0.75) and in methanol and CTAB micelles (0.76). Moreover, the ratio between FD of PN and FD of RB (FDPN/FDRB) was found to be constant and close to 1.3.
3.2 1O2 production by NSAID in microemulsions
The UV spectra of the NSAID in the different microheterogeneous systems (not shown) point to the oil-water interphase as their most likely location, which does not depend on the charge of the surfactant. We have determined the values of FD for different NSAID and derivatives in anionic and cationic microemulsions.
TA, SP and KP show lower FD values in cationic than in anionic microemulsions (see Table 1). These three compounds contain, in addition to the COOH group, a carbonyl group which, due to its polarity, could also be located close to the surfactant polar heads and to the water pseudo-phase. The other molecules showed the inverse trend (FD in cationic systems > FD in anionic systems, albeit not to a large extent). It is interesting to note that DTA (no COOH but still a carbonyl group) exhibits similar FD values in cationic and in anionic microemulsions. This fact suggests that the pKa values of the drugs may be altered by the interaction with SDS and CTAC, leading to higher decarboxylation rates in cationic microemulsion for TA, SP and KP.
The influence of the pH on the values of FD was assessed by measuring the efficiency of 1O2 production by some of the drugs in anionic microemulsions which were prepared using a phosphate buffer (pH 7.2) instead of water in the formulation. In addition, experiments were carried out in SDS micellar solutions in D2O and the results were compared with those obtained in pure D2O and D2O pD = 7.2. Results are summarized in Table 2.
Values of FD measured for TA are very sensitive to the pH: they decrease as the pH of the medium increases both in D2O and in the W/O anionic microemulsion. Despite the structural similarity between TA and SP, values measured for SP are not affected by pH (in the range investigated). It is noteworthy that, in both cases, a large increase of the FD value is observed when going from homogeneous or micellar solution to microemulsions, independently of the pH (see Tables 1 and 2). This shows that TA and SP are more protected from water in the microemulsion than in micellar media.
Values of FD found for NP are relatively insensitive to the pH. However, values in microemulsions are clearly higher than those measured in aqueous and micellar solution, as for TA and SP.
Although DTA does not contain a COOH group and is hardly soluble in water, an increase of FD was observed in microemulsions with respect to micellar solution (as for TA, SP and NP). Moreover, FD of DTA increases in the buffered W/O microemulsion compared to the non buffered one.
Further experiments on the
organization of the medium coupled with pH effects are currently in progress.
| NSAID |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
![]()
REFERENCES
[1] N.J. Turro, M. Grätzel, A.M. Braun, Angew.Chem. 19, 675-696 (1980)
[2] J.H. Fendler, J.Phys.Chem. 84, 1485-1491 (1980)
[3] L.A. Martínez, A.M. Braun, E. Oliveros, J.Inf.Rec., (1998) in press.
[4] M Boneva, S. K. Ivanov, E. Oliveros, A. M. Braun, J. Photochem. Photobiol. A: Chem, 68, 343, 1992.
[5] E. Oliveros, P. Murasecco-Suardi, A. M. Braun and H. J. Hansen, Efficiency of singlet oxygen quenching by carotenoids measured by near-infrared steady-state luminescence, in L. Parker (Ed.), Carotenoids, Methods in enzymology, Part A, Chemistry, Separation, Quantitation and Antioxidation, Academic Press, San Diego, CA, 1992, 420-429
[6] D. de la Peńa, C. Martí, S. Nonell, L. A. Martínez and M. A. Miranda, Time-resolved near infrared studies on singlet oxygen production by the photosensitizing 2-arylpropionic acids, Photochem. Photobiol. 65 (5) 828-832 (1997)
ACKNOWLEDGMENTS
L. A. M. gratefully acknowledges financial support provided by the ALEXANDER VON HUMBOLDT FOUNDATION.