Photochemical Consequences of Porphyrin and Phthalocyanine Aggregation on Nucleoprotein Histone
K. Langa*, P. Kubátb , J. Mosingera, D. M. Wagnerová a
a
Institute of Inorganic Chemistry, Academy of Sciences of the Czech Republic, 250 68 R ez , Czech Republic, lang@iic.cas.czb
J. Heyrovský Institute of Physical Chemistry, Academy of Sciences of the Czech Republic, Dolejš kova 3, 182 23 Praha 8, Czech Republic
________________________________________________________________________________
Anionic photosensitizers, meso-tetrakis(4-sulfonatophenyl)porphyrin (TPPS) and chloroaluminium phthalocyanine tetrasulfonate (AlPCS), are bound to histone by Coulombic attraction as has been evidenced by absorption and fluorescence spectra. At variance to serum albumins, histone promotes aggregation of the bound monomeric sensitizer, the apparent dimerization constants being KD = 4.2 x 105 M-1 (± 0.4 x 105) and 3.3 x 105 M-1 (± 0.7 x 105) for TPPS and AlPCS, respectively. Hydrophobic environment and the shielding effect of positively charged histone act as a self-aggregating driving force for the formation of stacked aggregates. Sensitizer-histone ground state interactions and their influence on photophysical properties have been analyzed. Reduction of the triplet quantum yields F T induced by aggregation on histone considerably decreases the sensitizing ability. The bimolecular rate constant for quenching of histone bound triplet 3TPPS by oxygen is (3.9 ± 0.7) ´ 108 M-1 s-1, which is one order of magnitude lower than that for the free sensitizer.
________________________________________________________________________________
Introduction
Application of singlet oxygen producing photosensitizers phthalocyanines and porphyrins in photodynamic therapy posed a question: How will proteins and DNA change photochemical and photophysical properties of the sensitizers and subsequent photoinduced reactions. Human and bovine serum albumins non-covalently bind anionic sensitizers like sulfonated porphyrins and phthalocyanines, and affect natural singlet and triplet lifetimes and rate constants of subsequent processes, as quenching of the excited triplet states by oxygen. Ground state interactions between the sensitizer and the protein have been shown to be essential for elucidation of these effects.
Nucleoproteins histones play a key role in the compaction of DNA. DNA is wound around a core of 8 histone units forming a DNA-protein complex called chromatin. With respect to their basic character, strong electrostatic attraction of anionic sensitizers can be expected. In spite of the evident importance of histones in the context of the photodynamic effect, their influence on exogenous sensitizers has not been reported so far.
Studied sensitizers:
5,10,15,20-Tetrakis(4-sulfonatophenyl)porphyrin tetrasodium salt, TPPS
5,10,15,20-Tetrakis(4-carboxyphenyl)porphyrin, TPPC
5,10,15,20-Tetrakis(4-N-methylpyridyl)porphyrin tetraiodide, TMPyP
chloroaluminium phthalocyanine tetrasulfonate (Porphyrin Products), AlPCS
Proteins:
Bovine serum albumin, BSA, (fraction V)
Arginine-rich histone H3 from calf thymus (Johns fraction III)
The combination of spectroscopy methods including UV/Vis, fluorescence, light-scattering, transient spectroscopy, laser-induced optoacoustic spectroscopy (LIOAS) and time-resolved luminescence detection of singlet oxygen was applied for characterization sensitizer-histone ground state interactions and their influence on photophysical properties.
For simplicity, sensitizer species are abbreviated without designation of oxidation states, charge, axial ligands, and in the case of sensitizer-protein adducts the formulas do not characterize the stoichiometry but denote the form of the sensitizer on the protein:
sensitizer-protein: is the adduct of monomeric sensitizer with the protein
sensitizeragg-protein: denotes sensitizer aggregates on the protein
Spectral changes induced by histone
Spectral features of the TPPS/histone system confirm binding of TPPS on histone and the presence of several spectroscopically distinguishable porphyrin forms. Increasing ionic strength leads to liberation of TPPS from histone adducts.
A parallel between the position and relative heights of absorption bands and fluorescence emission peaks of monomer bound to histone and to BSA shows that the hydrophobic microenvironment of both proteins has the same effect on spectral properties (Tab. 1):
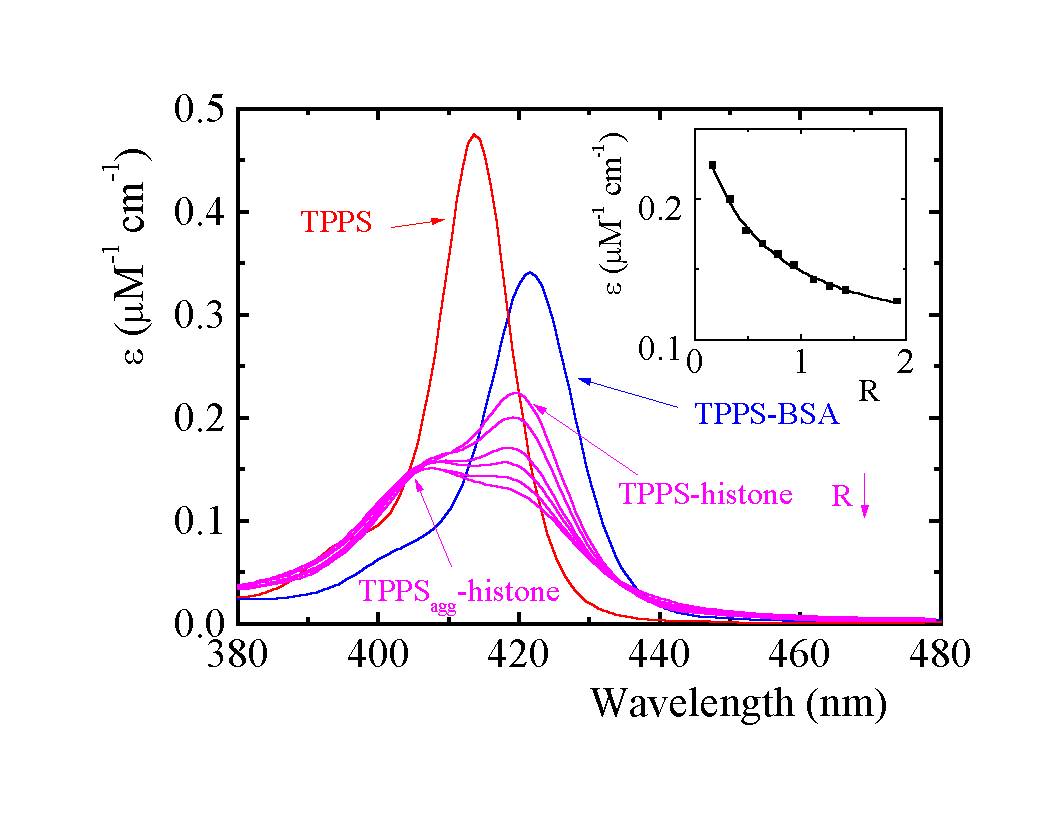
Fig. 1. Absorption spectra of TPPS in the Soret region: R (porphyrin/histone) = 0.16, 0.32, 0.64, 0.78, 1.27 and 1.90. With increasing R the observed e at 420 nm decreases (see inset). Phosphate buffer pH 5.70 (7 mM), 3.3 m M histone. Absorption spectra of 1.8 m M TPPS and of the TPPS-BSA adduct, R = 0.16.
Difference absorption spectra of sensitizer-histone were recorded against BSA solutions with equal concentration of the sensitizer in the form of the sensitizer-BSA adduct. The isosbestic points at 413 nm for TPPS/histone and at 599 nm, 614 nm, 662 nm, and 697 nm for AlPCS/histone indicate an equilibrium between two species. The subtraction of the monomer peak from the total envelope of absorption spectra enables spectral characterization of a second form which differs from bound monomer:
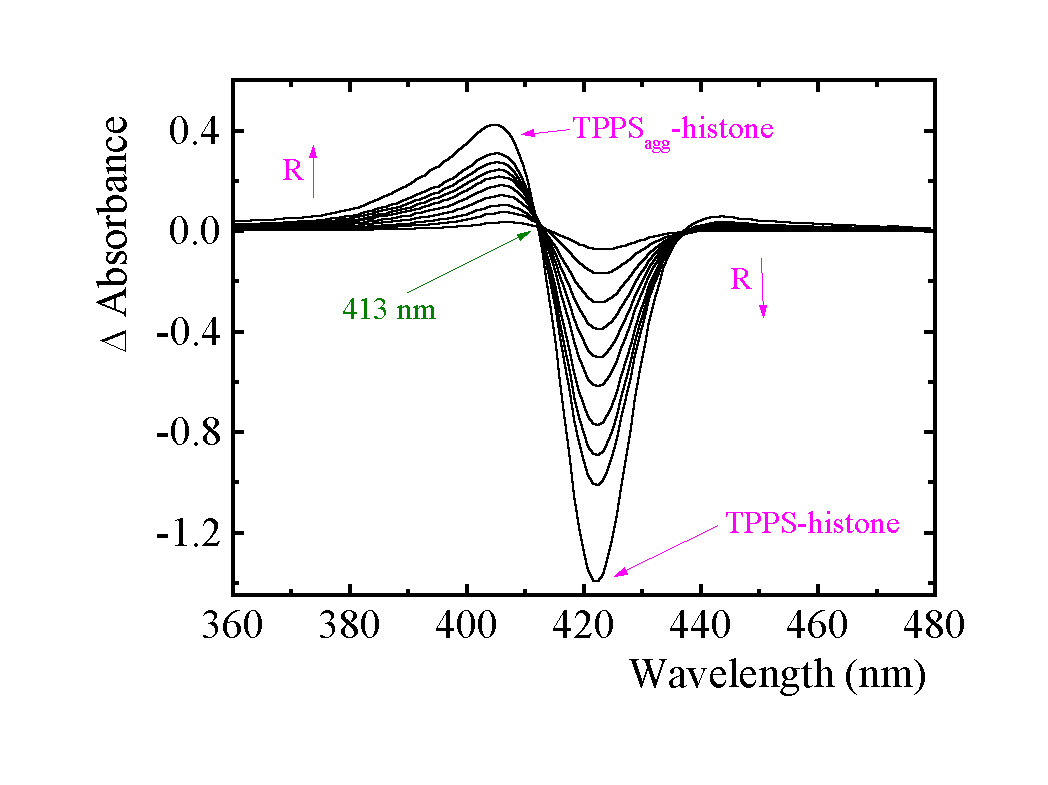
Fig. 2. Difference absorption spectra of TPPS in the presence of histone measured vs. monomer of TPPS bound to BSA (TPPS-BSA). Increasing R from 0.16 to 1.91 is indicated by the arrow. Phosphate buffer pH 5.70 (7 mM), ca 3.3 m M histone.
We have spectral evidence that aggregation of TPPS and AlPCS occurs on histone (see Table 1). The formation of extended sensitizer aggregates has been excluded by light scattering measurement. Presuming a dimerization model, one can obtain the apparent dimerization constants KD of 4.2 x 105 M-1 (± 0.4 x 105) and 3.3 x 105 M-1 (± 0.7 x 105) for TPPS and AlPCS, respectively. Though the model well describes the datasets, it simplifies the real behavior (corrections for local concentrations, aggregation number can depend on R).
Emission fluorescence spectra of TPPS attached to histone
At pH < 5.70, two porphyrin nitrogens of TPPS are protonated to form the dianion H2TPPS (pKa = 4.8). When histone is added, the emission peak at 680 nm (Fig. 3), belonging to protonated H2TPPS, is fully substituted by the TPPS-histone emission peaks - only unprotonated TPPS is accommodated.
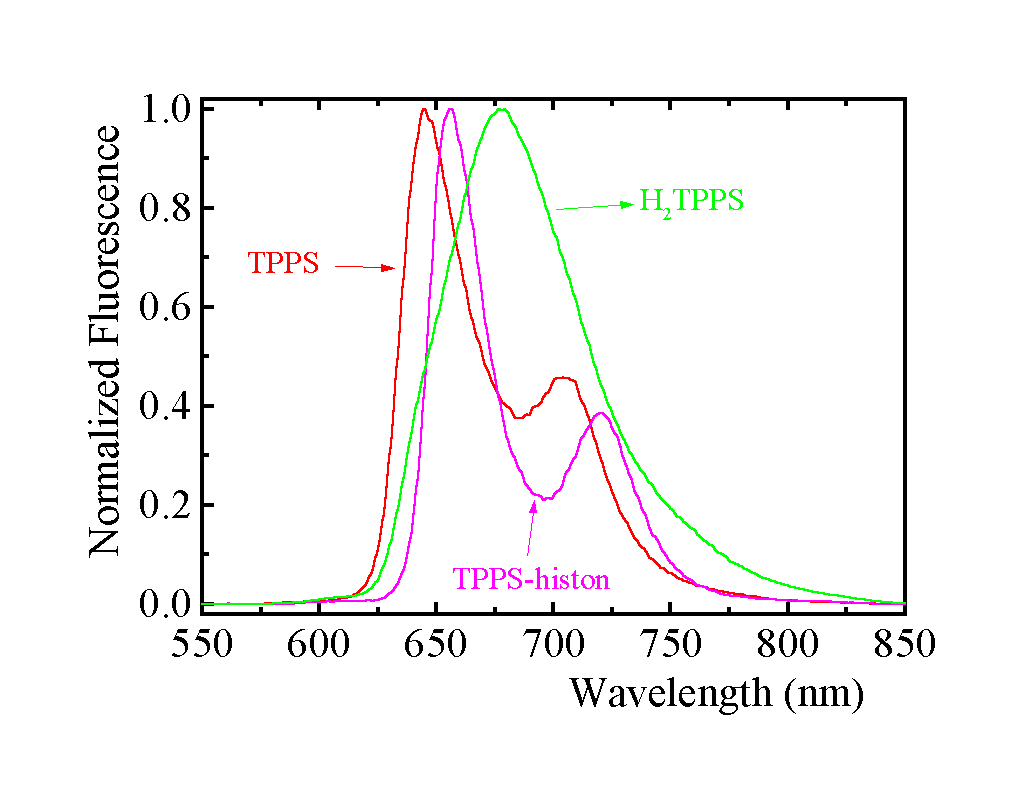
Fig. 3. Normalized fluorescence spectra of TPPS using l exc = 519 nm and of protonated H2TPPS using l exc = 430 nm measured for the same sample. The emission peaks in the presence of 3.3 m M histone using l exc = 510 nm are shifted; in general they are independent of excitation wavelength. Phosphate buffer pH 5.70 (7 mM), 1.8 m M TPPS.
Collected spectroscopic features of TPPS and AlPCS adducts (Table 1)
Compound |
Absorption spectrum l max /nm (log e /M-1 cm-1) |
Fluorescence spectrum l max /nm |
TPPS |
414 (5.65); 516 (4.20); 553 (3.83); 580 (3.81); 633 (3.57) |
646; 704 |
TPPS-histone |
519 (408); 555 (3.63); 593 (3.66); 650 (3.52) |
656; 722 |
TPPSagg-histone |
405a) |
not resolvedb) |
TPPS-BSA |
422 (5.53); 519 (4.20); 553 (3.91); 592 (3.78); 646 (3.59) |
653; 718 |
AlPCS |
351 (4.78); 607 (4.47); 674 (5.25) |
686; 747 |
AlPCS-histone |
350; 673 |
688; 755 |
AlPCSagg-histone |
627a); 656a) |
not resolved |
AlPCS-BSA |
351 (4.72); 609 (4.46); 676 (5.26) |
689; 752 |
a)
from difference absorption spectrab)
TPPS-histone has much higher F f than TPPSagg-histone. Very low fluorescence emission of TPPSagg-histone is probably at 680 nm and 750 nm as follows from excitation fluorescence spectra
Quenching of 3TPPS and 3TPPS-histone by oxygen
The kinetics of 3TPPS decay and of 3TPPS quenching by oxygen slows down after addition of histone. Even though the shape of triplet-triplet absorption spectrum is not changed, its intensity is considerably reduced with increasing R. It implies that the quantum yields of triplet formation F T decrease as a result of TPPS aggregation. Histone hinders protonation of the excited triplet states.
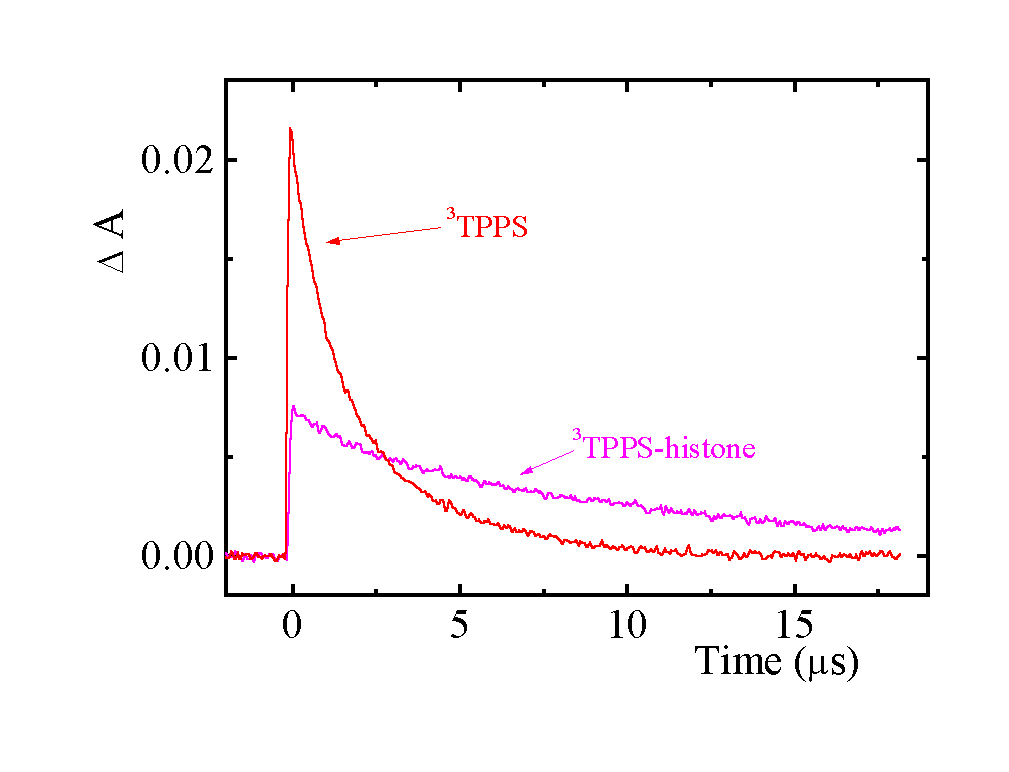
Fig. 4. Transient kinetic traces following 413 nm laser pulse excitation of 1.8 m M TPPS in the absence and in the presence of ca. 3.4 m M histone. Phosphate buffer pH 5.70 (7 mM), absorbance measured at 450 nm.
The quantum yields F T were measured using LIOAS. The quantum yields of singlet oxygen formation F D were estimated from luminescence of 1O2.
Excited state properties of TPPS in the presence of histone and BSA (Table 2).
histone |
BSA |
solution |
|
emission maximum Q(0,0) |
656 nm |
653 nm |
646 nm, 675 nma) |
fluorescence quantum yield |
0.035b) |
- |
0.060 ± 0.005c) |
triplet absorption maximum |
450 nm |
450 nm |
450 nm |
triplet quantum yields |
£ 0.2d) |
- |
0.76 ± 0.08e) |
triplet lifetime |
1 000 m sf) |
2 400 m sf) |
510 m sg) |
quantum yields of 1O2 |
< 0.1h) |
- |
0.57 ± 0.14i) |
kq (M-1s-1) |
(3.9± 0.7)x108 |
(4.7 ± 0.5) x 108 |
(1.8± 0.1)x109 |
a)
protonated H2TPPS. b) ± 10%, R = 0.55. c) T. Gensch and S. E. Braslavsky, J. Phys. Chem. B, 101 (1997) 101.d) R ~ 0.2. e) R. Bonnet, R. J. Ridge, E. J. Land, R. S. Sinclair, D. Tait and T. G. Truscott, J. Chem. Soc., Faraday Trans. I, 78 (1982) 127. f) error 20 %. g) P. Kubát, K. Lang, J. Mosinger and D. M. Wagnerová, submitted. h) R ~ 1. i) average literature value from F. Wilkinson, W. P. Helman and A. B. Ross, J. Phys. Chem. Ref. Data, 22 (1993) 113.
Conclusions
1. We present evidence that both TPPS or AlPCS are accommodated on histone and that the equilibrium is not established between free (uncomplexed) and bound sensitizer, as has been generally found for albumins.
2. We report the existence of two sensitizer-histone forms in equilibrium at low R ~ 0.1 i. e. under conditions where no aggregation on albumins has been observed. The monomer-histone adduct is expected to have spectral and photochemical properties similar to monomer attached to albumins. The second species behaves spectrally as aggregated TPPS or AlPCS. Apparently, monomer and aggregate are relatively freely attached to histone binding sites so that the equilibrium between them can be established.
3. The apparent dimerization constants of KD = 4.2 x 105 M-1 (± 0.4 x 105) and 3.3 x 105 M-1 (± 0.7 x 105) for TPPS and AlPCS, respectively, were obtained presuming a dimerization model. For comparison, TPPS in solution has KD of 4 x 103 M-1 [T. Gensch and S. E. Braslavsky, J. Phys. Chem. B, 101 (1997) 101.], i. e. two orders of magnitude smaller. AlPCS in aqueous solutions is believed to be monomeric.
4. Interaction with histone is governed by the sensitizer's negative charge. Both TPPS and AlPCS have peripheral sulfo groups and therefore the molecules are in fact tetravalent anions. Precipitation of histone occurring for R > 2 can be explained by protein compaction caused by the excess of the multifunctional sensitizers. Substitution of TPPS by TPPC bearing four carboxy groups does not affect the above described behavior. In contrast, positively charged porphyrin TMPyP does not show any sign of interaction with histone and of precipitation (examined up to R = 4.0).
5. Inhibition of TPPS protonation in the ground and triplet states by histone confirms that the sensitizers are placed in an environment with low activity of water molecules.
6. Quenching of fluorescence is not attributed to an enhancement of efficiency of intersystem crossing since in fact F T decreases. Instead, efficiency increase of internal conversion is suggested due to aggregation of the sensitizers within histone. The F T decrease implies considerable reduction of F D . Thus, the organization and distribution of non-covalently bound sensitizer molecules to histone considerably affect sensitizing ability via induced aggregation, quenching of F T and, consequently, F D .