FURTHER CHARACTERIZATION OF PERINAPHTHENONE (1H-PHENALEN-1-ONE) AS O2(1Dg) STANDARD: PHOTOCHEMICAL DECOMPOSITION IN 1,4-DIOXANE AND DMA
Esther Oliverosa, Stefan Bossmanna, Santi Nonellb, Cristina Martib,Gernot Heita, Gabi Tröschera, Annette Neunera, Claudia Martíneza and André Brauna.
a Lehrstuhl Umweltmesstechnik, Engler-Bunte-Institut, Universität Karlsruhe, D-76128 Karlsruhe (Germany)b Institut Químic de Sarrià, Universitat Ramon Llull, Via Augusta 390, 08017-Barcelona (Spain)
INTRODUCTION
1-H-Phenalen-1-one (perinaphthenone), an efficient singlet oxygen (O2(1Dg)) sensitizer with quantum yield close to unity, has been proposed as a universal standard sensitizer for O2(1Dg) studies[1-3]. However, data about chemical and photochemical stability of phenalenone is limited to a few solvents [4,5]. This parameter is of crucial interest for singlet oxygen studies under CW irradiation over long periods of time. In this contribution, we present experimental results demonstrating that, despite low quantum yields, significant photochemical decomposition of phenalenone occurs under steady-state irradiation in air equilibrated 1,4-dioxane and N,N’-dimethylacetamide (DMA), mainly due to hydrogen abstraction from the solvent by its triplet excited state. Despite these photoreactions, the quantum yield of singlet oxygen production, determined using time-resolved near-IR emission [6] and laser-induced optoacoustic calorimetry (LIOAC) [7], remains high.
The results for phenalenone (PN) are compared to those for 9-H-fluoren-9-one (FLU), a photochemically stable ketone.
RESULTS
|
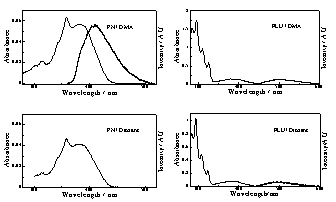
Fluorescence PN is non-fluorescent in 1,4-dioxane and slightly fluorescent in DMA, FF = 0.008. The same solvent trend is observed for FLU, FF = 0.008 in 1,4-dioxane and FF = 0.024 in DMA, for which this sovent effect has been attributed to re-ordering of the np* and pp* excited states. |
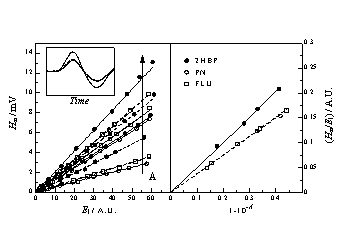
| Figure 2 (left panel) shows the zero-time intensity of the 1270 nm singlet oxygen phosphorescence plotted as a function of the laser energy for PN and FLU solutions of increasing absorbance in 1,4-dioxane. In the right panel, the slopes of the straight lines thus obtained ar plotted vs the absorption factor. The ratio of slopes shows that the FD values for PN and FLU are in the ratio 0.93 in 1,4-dioxane. In DMA the ratio goes down to 0.73. | 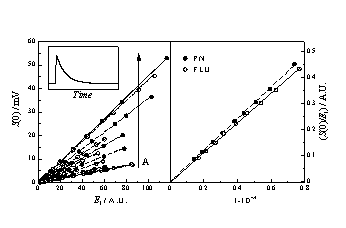 |
|
1,4-dioxane Figure 3 shows the maximum amplitude of the optoacoustic wave as a function of the energy of the laser pulse for PN and FLU oxygen saturated solutions of increasing absorbance and dependence of the slopes of the straight lines thus obtained on the absorption factor. Equations: Hm = C’ a El (1-10-A) and FDED = (1-a) Eexc - FfEf The absolute FD values obtained for PN and FLU are 0.99 and 0.94 respectively. The ratio between them is 0.95, as with TRPD. |
| DMA In this solvent, an additional solvent-oxygen charge transfer band competes for light absorption with absorbance ACT (at 337 nm). Thus:
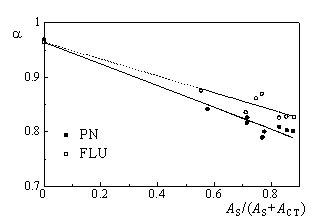
Figure 4 shows the dependence of the fraction of absorbed laser energy released as prompt heat ( a) on the relative absorbance of the sensitizer S (AS/(AS+ACT)) in oxygen saturated DMA solutions.The absolute FD values for PN and FLU in this solvent are 0.87 and 0.66, respectively. The ratio between them is equal to 0.75, in very good agreement with the values obtained with TRPD. |
|
 |
Decomposition
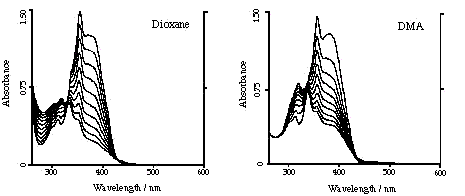
of PN under continuous irradiation The decrease of the UV/VIS absorbance under continuous irradiation of PN solutions at 367 nm in air equilibrated DMA and 1,4-dioxane, are shown in the figure (absorption spectra registered before irradiation and every 5 min up to 45 min of irradiation are represented). It can be observed that PN is not photochemically stable in these solvents under continuous irradiation |
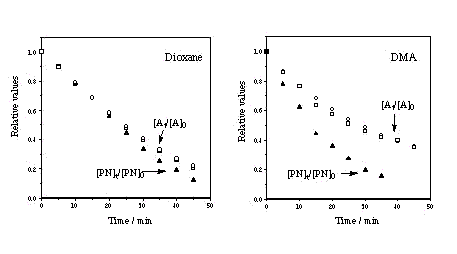
| PN disappearance Measurement of the decrease of the relative PN concentration ([PN]t/[PN]0, full triangles) and of the relative absorbance at 367 nm (At /A0 , circles: same solution irradiated during 45 min; squares: solutions used for HPLC analysis) under continuous irradiation of air equilibrated DMA and 1,4-dioxane solutions (lex = 367 nm, A0 =1.24) Since PN is inert towards singlet oxygen, the most probable mechanistic hypothesis to explain the PN disappearance is that hydrogen abstraction from the solvent by the PN triplet excited state competes with 1O2 production. |
 |
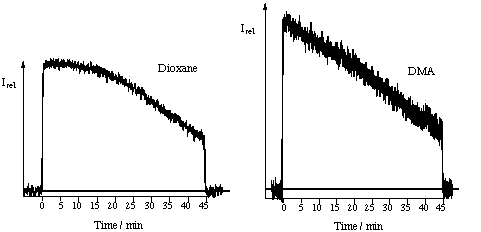 |
Singlet
oxygen production under continuous irradiation Singlet oxygen NIR phosphorescence signals under continuous irradiation of PN solutions at 367 nm (time zero corresponds to the beginning of the irradiation, signals have been recorded at different sensitivities in DMA and dioxane). An evaluation of the relative FD of FLU and PN in DMA and dioxane may be obtained from the ratio of the 1O2 NIR signals under steady-state irradiation. Ratios of 0.93 and 0.73 have been found in dioxane and DMA, respectively, in very good agreement with TRPD.If PN reaction products act only as inner filters, we can assume:
|
| Measuring the relative singlet oxygen NIR phosphorescence signal (Se,t/ Se,0,, full triangles) and the relative concentration of singlet oxygen calculated according to the equation above ([1O2]t / [1O2]0, circles) as a function of the irradiation time of PN solutions (figure on the right), we conclude that the singlet oxygen production under continuous irradiation decreases more slowly than predicted for equation. At least one of the PN reaction products is also a singlet oxygen sensitizer. Phenalanone and 7,8-dihydroacenaphtene have been identified by GC-MS/FTIR | 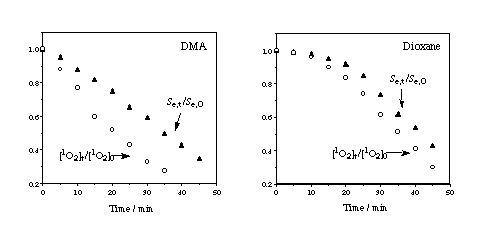 |
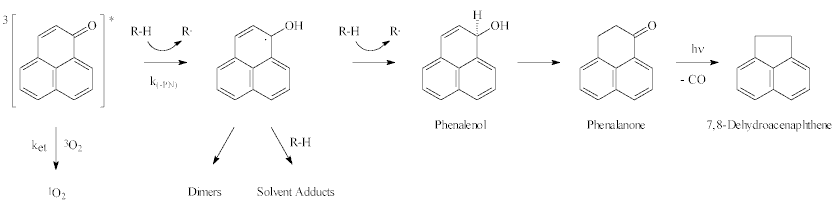
| A
possible mechanism for PN disappearance is shown in this scheme.
|
|
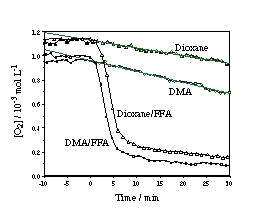
| The figure shows the evolution of the concentration of dissolved oxygen measured with an oxygen optical sensor in air equilibrated PN solutions: In DMA (full squares); in DMA in the presence of FFA (open squares); in 1,4-dioxane (full triangles); in 1,4-dioxane in the presence of FFA (open triangles). The results are collected in table 1, and we can conclude that: (a) the molar ratio of PN and 3O2 consumed depends on the relative efficiencies of the various reaction paths in the scheme and (b) there might be a competing H-abstraction from FFA by the PN triplet excited state leading to formation of radicals and, thus, increased oxygen consumption. |
|
Table 1: Results under CW irradiation
| System | [PN] [mol L -1] |
[FFA] [mol L -1] |
F (-PN) |
| PN/DMA/hn | 1.46x10 -4 |
0 |
2.1(±0.2)x10 -2 |
| PN/1,4-dioxane/hn | 1.40x10 -4 |
0 |
1.3(±0.1)x10 -2 |
| PN/DMA/FFA/hn | 1.46x10 -4 |
10 -2 |
~ 2.5x10 -5 |
| PN/1,4-dioxane/FFA/hn | 1.40x10 -4 |
10 -2 |
~ 10 -5 |
System |
- d [ 3O2]/dt[ mol L-1 s -1] |
F D |
F (-3O2) |
PN/DMA/FFA/h n |
4.2(±0.4)x10 -6 |
1.02 ± 0.20 |
1.02 ± 0.20 ([FFA] ~ 10 -2 mol L-1) |
PN/1,4-dioxane/FFA/h n |
4.3(±0.4)x10 -6 |
1.05 ± 0.20 |
1.05 ± 0.20 ([FFA] ~ 10 -2 mol L-1) |
PN/DMA/h n |
0.19(±0.01)x10 -6 |
- |
4.6(±0.6)x10 -2 |
PN/1,4-dioxane/h n |
0.14(±0.01)x10 -6 |
- |
3.4(±0.4)x10 -2 |
CONCLUSIONS
1. The quantum yields of singlet oxygen production by PN in DMA and 1,4-dioxane are 0.87 and 0.99 respectively, determined by TRPD and LIOAC, confirming that this compound is a very efficient 1O2 sensitizer. The value for DMA is lower than in most solvents.
2. The quantum yield of singlet oxygen production for FLU in DMA is lower than in 1,4-dioxane (0.66 and 0.94). This is in agreement with previous results showing that
fD for FLU decreases with increasing solvent polarity.3. Although PN is stable under time-resolved irradiation with laser pulses of very low energy, it decomposes under steady-state irradiation, in air-equilibrated 1,4-dioxane and DMA, mainly due to hydrogen abstraction from the solvent by its triplet excited state..
4. Values of 2.1x10-2 and 1.3x10 -2 have been obtained for the the quantum yields of disappearance of PN in DMA and 1,4-dioxane, respectively. Although these values are very small, they lead to an important decrease of the absorbance observed at 367 nm during irradiation.
5. Phenalanone and 7,8-dihydroacenaphtene, have been identified among the reaction products in both DMA and 1,4-dioxane by GC-MS/FTIR. A mechanism for the formation of these products is proposed.
REFERENCES [1]E. Oliveros, P. Suardi-Murasecco, T. Arminian-Saghafi, A. M. Braun and H.-J. Hansen, Helv. Chim. Acta, 1991, 74, 79-90.[2]R. Schmidt, C. Tanielian, R. Dunsbach and C. Wolff, J. Photochem. Photobiol., A: Chem., 1994, 79, 11-17.
[3]C. Martí, O. Jürgens, O. Cuenca, M. Casals and S. Nonell, J. Photochem. Photobiol. A:Chem., 1996, 97, 11-18.
[4](a) H. Köller, G. P. Rabold, K. Weiss and T. K. Mukherjee, Proc. Chem. Soc., 1964, 332-336.(b) G. P. Rabold, K. H. Bar-Eli, E. Reid and K. Weiss, J. Chem. Phys., 1965, 42, 2438-2447.
[5]N. A. Kuznetsova, A. V. Reznichenko, V. N. Kokin and O. L. Kaliya, J. Org. Chem. USSR (Engl. Transl.), 1982, 18, 540-544.
[6](a) A. U. Khan, Chem. Phys. Lett., 1980, 72, 112-114.(b) A. A. Krasnovsky Jr., Chem. Phys. Lett., 1981, 81, 443-445.(c) M. A. J. Rodgers, J. Am. Chem. Soc., 1983, 105, 6201-6205.(d) A. M. Braun, and E. Oliveros, Pure Appl. Chem., 1990, 62, 1467-1476. (e) S. Croux, M.-T. Maurette, M. Hocquaux, A. Ananides, A. M. Braun and E. Oliveros, New J. Chem., 1990, 14, 161-167.(f) E. Oliveros, F. Besançon, M. Boneva, B. Kräutler and A. M. Braun, J. Photochem. Photobiol., B: Biol., 1995, 29, 37-44.
[7]S. E. Braslavsky and G. E. Heibel, Chem. Rev., 1992, 92, 1381-1410
![]()
Web Designer: Javier Belmar
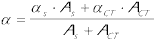
 .
.