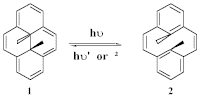Investigating the Photochromism of the Dimethyldihydropyrene System R. Scott Murphy, Y. Chen, Reginald H. Mitchell, and C. Bohne, Department of Chemistry, University of Victoria, P.O. Box 3065, Victoria, B.C., V8W 3V6
Successful applications of photochromic compounds relies on a clear understanding of the mechanism. Dimethyldihyropyrene (1) is a fully delocalized bridged [14]annulene system which undergoes a reversible photoisomerization when irradiated with visible light to give the ring-opened cyclophanediene (2). The reverse reaction can occur either photochemically or thermally. Laser flash photolysis has been employed to study the intermediate involved in the ring-opening. Quenching studies suggest that the intermediate is a triplet diradical. Studies into the benzo[a]- and benzo[e]-fused derivatives of 1 are currently in progress.