The Photochemistry of Trimethyltriphenylgermasilanes
|
Nicholas P. Toltl, William J. Leigh, and Keith H. Pannell Mcmaster University, Department of Chemistry, 1280 Main St. W. Email : toltlnp@mcmaster.ca |
![]()
Silenes and Germenes
| Theory at the ab initio level has described some of the characteristics of alkenes, silenes, and germenes. As expected, ethene does not have a polarized double bond and the bond length is 15% shorter than that of a C-C single bond. Silicon-carbon and germanium-carbon double bonds are polarized and the bond length gets increasingly longer as you go from carbon to silicon to germanium |
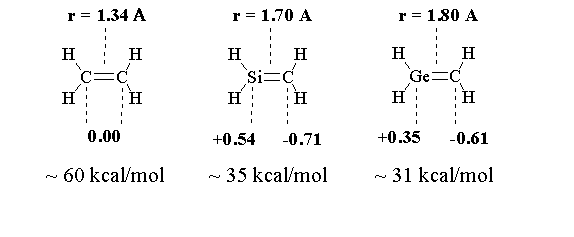
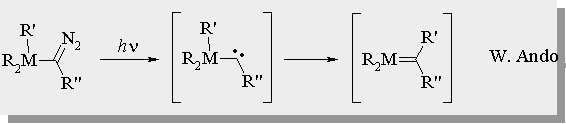
| There are many examples for the generation of silenes and germenes in the literature |
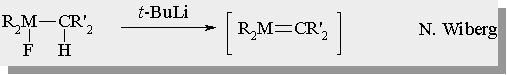
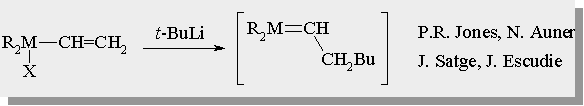
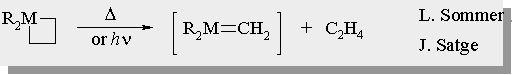
Simple Si=C and Ge=C Reactivity
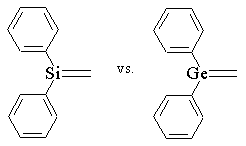
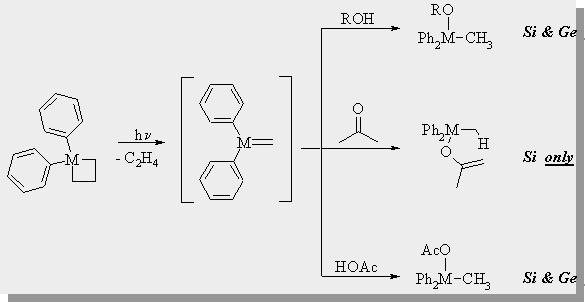
| In our lab, we have investigated the differences in reactivity of 1,1-diphenylsilene and 1,1-diphenylgermene which are produced photochemically from 1,1-diphenylmetallocyclobutane. Below is a summary of the reactivity differences between the 2 compounds. |
Toltl, N.P.; Leigh, W.J.; J. Am. Chem. Soc., 1998, 120, 1172-1179
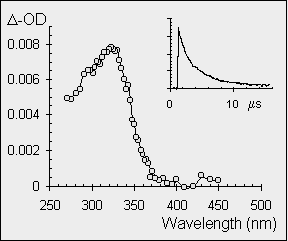
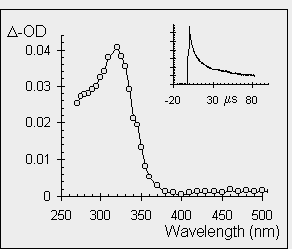
both of these transient species (silenes and germenes) have been studied by nanosecond laser flash photolysis techniques. The insert shows the decay kinetics of the transient observed at 320 nm and it is evident that the lifetime of diphenylgermene is longer than that of diphenylsilene. The spectrum below shows the absorbance plotted against wavelength in a point by point fashion with the absorption maximum for both diphenylsilene and diphenylgermene occurring at 320 nm. |
Ph2Si=CH2 Ph2Ge=CH2
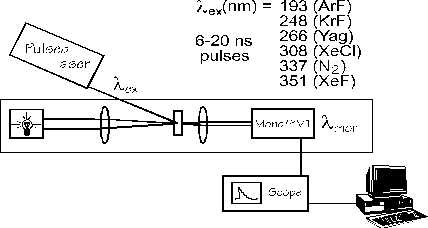
| below is a schematic representation of our nanosecond laser flash photolysis system. The use of various gas mixtures in the laser can generate different wavelengths while the rest of the system is basically a large UV spectrometer. The monochromator can be set to monitor the absorbance of a transient at a particular wavelength and this absorbance is measured over time. The resulting decay trace is analyzed through the use of a computer and decay kinetics are then determined. |
Nanosecond Laser Flash Photolysis
| These are the absolute rate constants in hexane (230C) of diphenylsilene and diphenylgermene with a variety of chemical traps. |
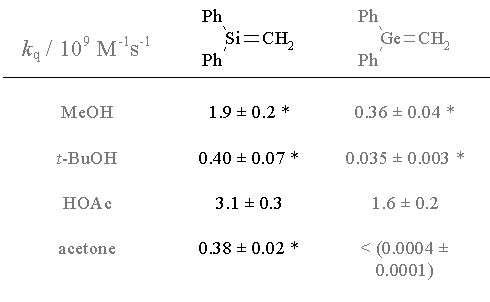
* k
H/kD 1.3-2.3Toltl, N.P.; Leigh, W.J.; J. Am. Chem. Soc., 1998, 120, 1172-1179
Disilane Photochemistry
| Irradiation of the disilane causes a formal [1,3-silyl] migration resulting in a transient 1,3,5-(1-sila)-hexatriene in >95% chemical yield. The transient absorption spectra of this transient is also shown. |
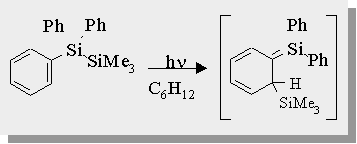
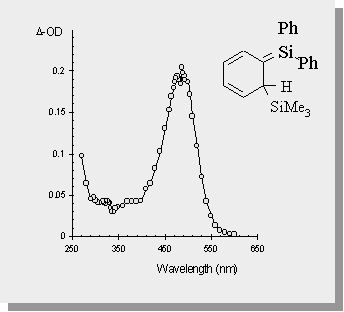
Leigh, W.J.; Sluggett, G.W.; Organometallics, 1994, 13, 269-281
| Trapping the 1,3,5-[1-sila]hexatriene with acetone affords 2 products - 1,2-siloxetane and a silyl ether and both of these compounds have been isolated and fully characterized. |
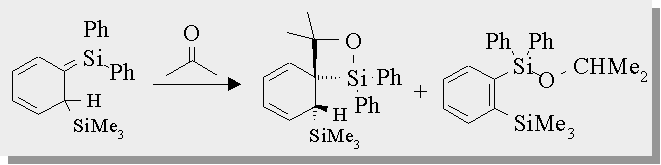
Toltl, N.P.; Leigh, W.J.; Organometallics, 1996, 15, 2554-2560
Digermane Photochemistry
| It is reported in the literature that the irradiation of digermanes affords quantitative Ge-Ge bond homolysis |
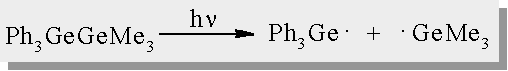
| Germyl radicals are trapped with CCl4 yielding the corresponding chlorogermanes |
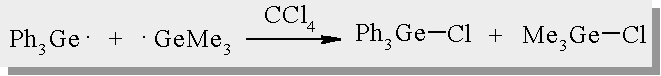
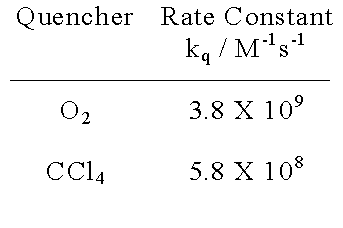
| The reactivity of triphenylgermyl radicals towards various quenchers has been reported |
Germasilane Photochemistry
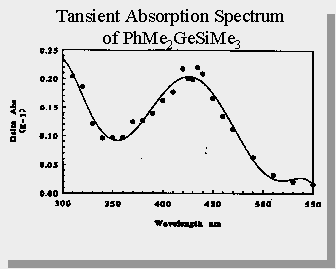
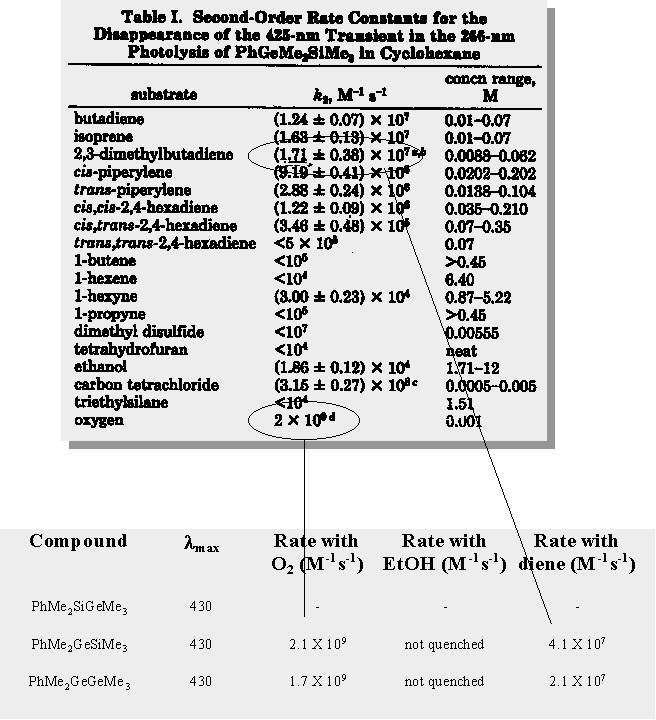
| PhMe2GeSiMe3 forms 2 transients upon photolysis - dimethylgermylene and a 1,3,5-(1-germa)-hexatriene |

Trapping experiments show that the ratio of germylene to germene is approx. 2:1

Laser flash photolysis studies on these germasilanes have been performed by both Gaspar (and coworkers) & Mochida (and coworkers). Both groups get similar results and characterize the transient occuring at 420 nm as being dimethylgermylene.
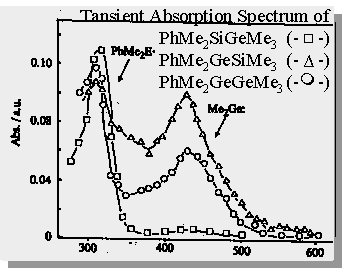
Gaspar, P.P. et. al.; Organometallics., 1991, 10, 2772-2777 Mochida, K. et. al.; Journal of Organometallic
Chemistry,1991,412,9-19
Germasilane Photochemistry
Gaspar, P.P. et. al.; Organometallics., 1991, 10, 2772-2777
Mochida, K. et. al.; Journal of Organometallic Chemistry,1991,412,9-19
Objectives
| to investigate the photochemistry of Ph3SiGeMe3 and Ph3GeSiMe3 through the use of product studies and nanosecond laser flash photolysis techniques | |
| attempt to study germylenes by NLFP techniques and compare the results to those already reported in the literature | |
| comment on the assignment (by other groups) of the transient monitored at 430 nm generated from the photolysis of PhMe2GeSiMe3 as being dimethylgermylene and not a 1,3,5-(1-germa)hexatriene |
Steady State Photolysis
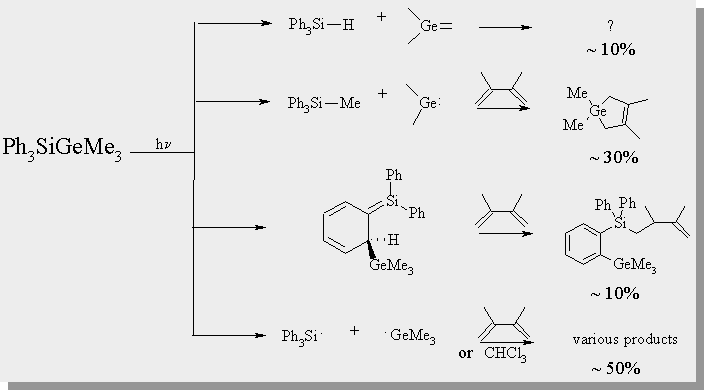
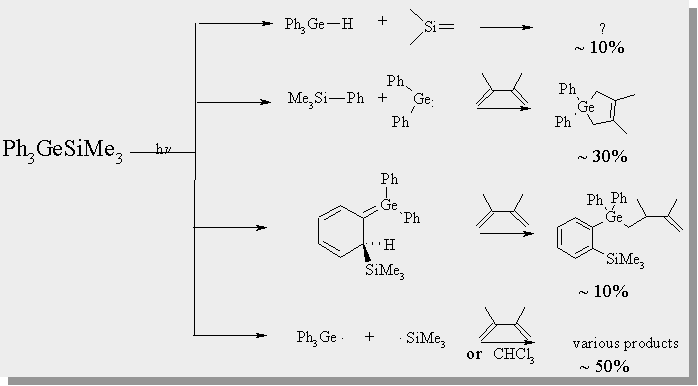
| the photolysis of both of the germasilanes affords numerous products. GC/MS and 1H NMR have allowed for a number of the products to be characterized and analyzed quantitatively. The relative ratio of each product is given below. |
Transient Absorption Spectra

| the transient absorption spectra of the germasilanes are shown below along with the spectrum of Ph3SiSiMe3 and one of the germasilanes in a polar solvent (acetonitrile). The transient at 490 nm is the result of the formal [1,3-metallo] migration into the phenyl ring yielding a transient 1,3,5-(1-metallo)hexatriene. The sharp absorption at 330 nm has been attributed to the Ge-Si bond homolysis affording germyl- or silyl- radicals. | |
| The photolysis of Ph3SiSiMe3 affords no signal that can be attributed to silyl radicals. | |
| The transient absorption spectra of the germasilanes in MeCN yields only peaks due to germyl- or silyl- radicals. This is because MeCN is a polar solvent which enhances intersystem crossing to the excited triplet state of the germasilane. It is known that Si-Si bond homolysis occurs from the triplet state of disilanes and therefore it is not surprising that similar results are seen in the germasilane case. |
Ph
3SiGeMe3 (hex) Ph3GeSiMe3 (hex)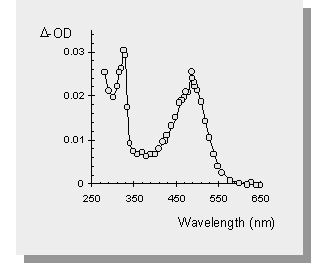
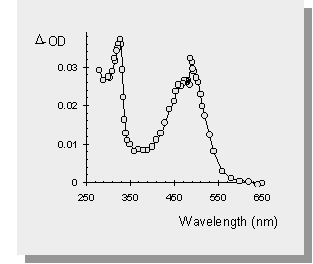
Ph
3SiSiMe3 (hex) Ph3GeSiMe3 (MeCN)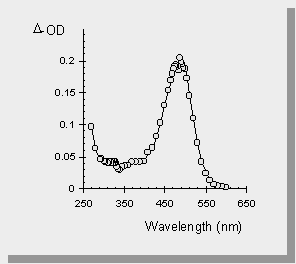
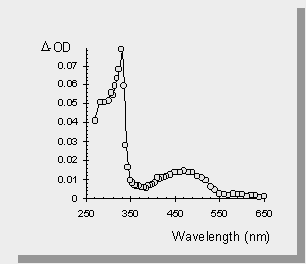
Absolute Rate Constants
| this is a comparison of the absolute rate constants for the 3 hextriene transients in hexane. |
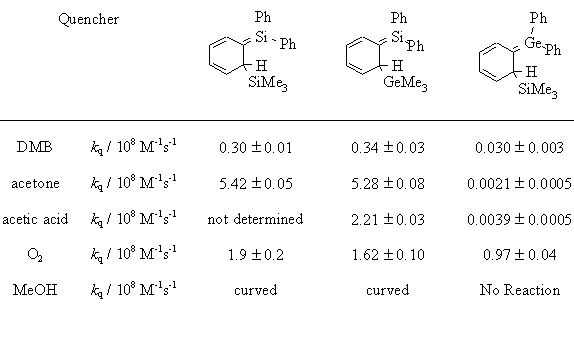
| absolute rate constants are determined by measuring the lifetime of a transient as a function of quencher concentration. A plot of kdecay vs. quencher concentration gives a straight line (in most cases) and the slope of the line is the absolute rate constant for that particular quencher. |
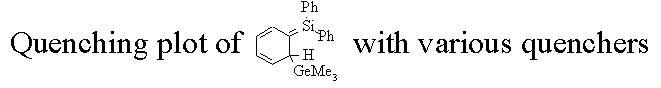
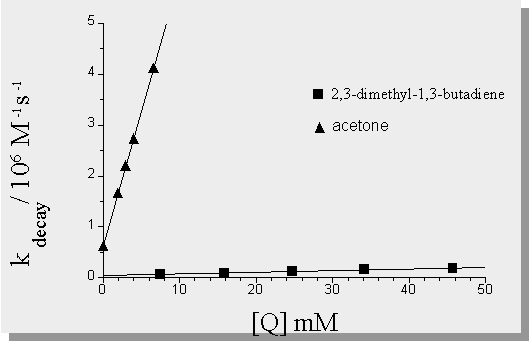
Discussion
| steady-state photolysis of the germasilanes affords a mixture of transient species |
| the extrusion of a germylene is the major pathways |
| products associated with a formal [1,3-metallo]- shift resulting in the hexatriene are also obvious |
| one transient (Me2M=CH2) is derived from a concerted dehydrosilylation mechanism |
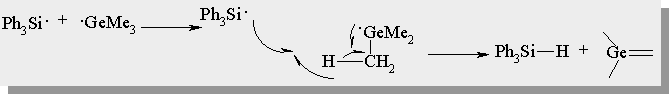
| nanosecond laser flash photolysis affords only 2 transient absorptions: hexatriene (lmax = 490 nm) and radicals (lmax = 330 nm) |
| no germylene absorption could be detected |
| radical formation is suppressed in the presence of 2,3-dimethyl-1,3-butadiene |
Conclusion
| the dominant reaction pathway of Ph3SiGeMe3 & Ph3SiGeMe3 is the extrusion of a germylene |
| other excited singlet state pathways are a formal [1,3-metallo] shift and a concerted dehydrosilylation reaction yielding dimethylsilene/germene |
| radical formation is derived from the excited triplet state of the germasilane |
| the presence of germylenes is confirmed by product studies and could not be observed using NLFP techniques |
Acknowledgements
Bruce Cook Ed Lathioor
Tracy Morkin Tom Owens
Jennifer Schmeisser Xian-Fu Zhang
$$$ NSERC $$$