On the Mechanism of Organic Azides Photooxidation
On the Mechanism of Organic Azides Photooxidation
On the Mechanism of Organic Azides Photooxidation
S.V.Zelentsov, A.B.Zhezlov, A.V.Oleynik
Nizhnii Novgorod State University, Chem.Department,
Gagarin Ave., 23. Nizhnii Novgorod 603600 Russia
E-mail: zelen@ichem.unn.runnet.ru
Photooxidation of organic azides is one of the least studied reaction. Although several mechanisms have been proposed [1-7] none of them is satisfactory to describe experimental data.
Abramovitch and others [1,2] seem to be the first chemists who began systematical study of the photooxidation mechanism. They have established that photooxidation products were nitro-, azoxy- and nitrosocompounds; azoxycompounds being formed due to the reaction
![]() (1)
(1)
Almost at the same time Brinen and Singh found [3] two possible intermediates that were involved in the reactions. One of them was the singlet nitrene-oxygen adduct, RNOOS, the other one was the triplet adduct, RNOOT.
RN + O2T
® RNOOS T S T ® RNOOS and RNOOT (2)The fate of the singlet adduct was studied by Pritchina and Gritsan [4]. They have proposed that RNOOS molecules are dimerized upon heating the reacting system up to 95 K, the dimer formed having the tetraoxide structure such as
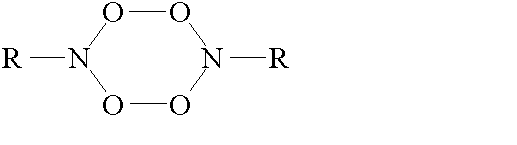
The tetraoxide decomposes to form nitro- and nitrosocompounds.
The fate of the triplet adduct was studied by Sawaki et al. [5]. They have revealed that RNOOT can oxidize such compounds as Ph2S, PhNO, C6H6, to form Ph2SO, PhNO2 and PhOH, respectively; RNOOT being "electrophilic oxygen transfer".
The solid state photooxidation mechanisms were proposed by some workers [6,7]. Treuschnikov, Frolova and Oleynik observed [6] formation of very stable products having asymmetrical ESR spectra. They proposed that in polymer matrices RNOOT can react with C-H bonds of polymer molecules to form pairs of radicals:
RNOOT
RNOOT
RNOOT
RNOOT + P-Í ® RN(H)OO· + P· (3)
The two radicals can stabilize themselves by reversible reactions, the ESR spectrum being superposition of asymmetrical peroxide radical and symmetrical (hydrocarbon) polymer radical.
RN(H)OO
RN(H)OO
RN(H)OO
RN(H)OO· + P· ![]() RN· (H) + POO· (4)
RN· (H) + POO· (4)
We have studied qualitative and quantitative composition of products formed in the course of the solid state (in polysterene films) and liquid phase (in benzene) photooxidation of 4,4'-diazidobiphenyl (1), 4,4'-diazidobiphenyl ester (2) and 1,4-diazidobenzene (3). We used non-filtered u.v. light irradiation (mercury 120 w lamp of super high pressure) of low intensity (of 20 w· m-2). We used thin layer chromatography (TLCG) and absorption spectroscopy to determine their yields. Table 1 shows RF values of the products.
Table 1.
RF valued of TLCG- separation of the photooxidation products.
|
¹ |
Rf values |
identification |
|||
|
spot |
11 |
21 |
31 |
12 |
|
|
1. |
0.97± 0.01 |
0.98± 0.01 |
0.99± 0.01 |
0.92± 0.02 |
azide used |
|
2. |
0.86± 0.04 |
0.85± 0.04 |
0.88± 0.02 |
0.60± 0.04 |
nitrosocompound |
|
3. |
- |
- |
- |
0.35± 0.03 |
nitrocompound |
|
4. |
0.73± 0.05 |
0.70± 0.05 |
0.81± 0.06 |
- |
azoxycompound |
|
5. |
- |
0.40± 0.04 |
- |
- |
carbonyl containing compound |
|
6. |
0.30± 0.1 0.15± 0.09 |
- |
0.20± 0.11 |
0.05± 0.04 |
oligomeric azocompounds |
|
7. |
0.00 |
0.00 |
0.00 |
0.00 |
azopolymer |
1)
1) in polysterene matrices
2)
in benzene 25oC, 0.01 M/l.Identification of the products consisted of their TLCG separation and application of the well-known analytical reactions. In addition all products involved in photooxidation process were synthezied and their RF -values were determined.
The results gathered in Table 1 forced us to conclude that solid state and solution photooxidation processes gave different products. We were not able to find nitrocompounds in polymer matrices, although they were the main products in benzene solution photoreaction. The main products of solid state photolysis are nitrosocompounds; their yields are only slightly depended on the chemical nature of the azide used (see Table 2).
Table 2
The yields of the photooxidation products (mass.%)
|
the substance |
polysterene films |
benzene |
||
|
|
1 |
2 |
3 |
1 |
|
nitrosocompound |
52.5 |
48.6 |
45.0 |
5.6 |
|
azoxycompound |
4.0 |
3.5 |
4.1 |
- |
|
nitrocompound |
- |
- |
- |
13.7 |
|
azocompounds |
15.0 |
15.0 |
18.0 |
28 |
|
conversion, % |
25.6 |
28.0 |
25.6 |
25 |
We have studied also the chemical nature of the radical products produced in the course of solid state photooxidation of organic azides [8,9]. Here we show a summary of our results. Fig.1 shows typical ESR signal of stable (at 25oC) paramagnetical products of azides photooxidation in solid polymer matrices and in the crystalline state.
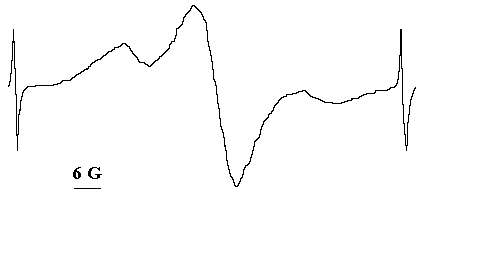
Fig.1 The typical ESR signal of the solid state photooxidation products [8].
We have adopted the model with g-factor and hyper fine coupling constants anisotropy to interprete the ESR spectra. Table 3 contains some of our results.
Table 3
g-Factors and super fine compling constants of the paramagnetical products of the solid state photooxidation
|
azide |
gzz |
gyy |
gzz |
Azz, G |
Ayy, G |
Axx, G |
|
11 |
2.0027 ± 0.0004 |
2.0059 ± 0.0005 |
2.0086 ± 0.0007 |
25.7 ± 0.3 |
5.0 ± 0.2 |
4.5 ± 0.2 |
|
12 |
2.0028 ± 0.0005 |
2.0055 ± 0.0004 |
2.0084 ± 0.0006 |
24.7 ± 0.3 |
4.6 ± 0.3 |
4.5 ± 0.4 |
|
22 |
2.0023 ± 0.0003 |
2.0054 ± 0.0004 |
2.0087 ± 0.0003 |
25.0 ± 0.3 |
4.8 ± 0.3 |
5.4 ± 0.4 |
|
32 |
2.0044 ± 0.0003 |
2.0068 ± 0.0003 |
2.0094 ± 0.0003 |
20.2 ± 0.3 |
4.0 ± 0.3 |
4.6 ± 0.3 |
|
Ph2NO · 3) |
2.0022 |
2.0056 |
2.0092 |
23.8 |
3.6 |
1.9 |
|
TMOPO4) |
2.0027 |
2.0061 |
2.0089 |
33.4 |
4.8 |
4.8 |
1)
1) 1) - in polysterene films; 2)
3)
- the data taken from [10];4)
- 2,2,6,6-tetramethyl-4-oxopyperidine-1-oxide in tetramethylcyclobutadione solution (the data taken from [11]).The results obtained have given us a possibility to proposed that iminoxide radicals are main paramagnetic products. Our ESR spectrum saturation experiments [9] have shown that iminoradicals are the only radical products; they being bound to macromolecules or located on the crystal surface.
We have assumed the following mechanism of the photooxidation of organic azides.
The nitrenes generated in the course of azides photolysis can react with oxygen to produce the corresponding adducts [3]:
RNS + O2T
T T T ® RNOOT (4)RNT + O2T
RNT + O2T
RNT + O2T
RNT + O2T ® RNOOS* S* * S Ph
Nitrosocompounds being the well-known spin traps are able to react with macroradicals or radical centers localized at crystall surfaces. The result of the reaction is production of iminoxides radical centers bounded to macromolecules or localized at the crystall surface.
RNO +
RNO +
RNO +
RNO + · P ® RN(O· )P )P )P )P (9)
The vibrationally excited singlet adduct RNOOS can give nitrocompound upon u.v. irradiation or give RNOOT as a result of the intersystem crossing process.
RNOOS*
RNOOS*
RNOOS*
RNOOS* ® RNO2 RNO2 RNO2 RNO2 (10)
The next route of the RNO2 formation is oxidation of RNO to RNO2 with RNOOT molecules.
To make it clear the mechanism of the RNO2 production we investigated nitrosocompounds oxidation to nitrocompounds. The fig.2 and 3 show that nitrocompounds successfully formed upon u.v. irradiation of nitrosocompounds in benzene solution saturated with O2, and the latter reaction being inhibited in solid polymer films. It might be the consequence of the reaction proposed by japanese workers [5] such as
RNO + RNOOT
RNO + RNOOT
RNO + RNOOT
RNO + RNOOT ® RNO2 RNO2 (11)
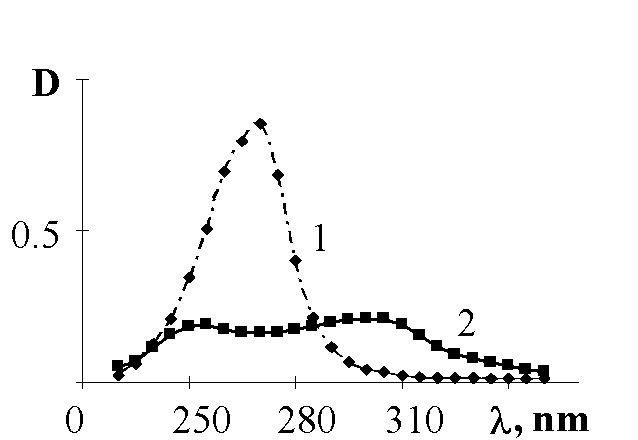
Fig.2. Photooxidation of 4,4'-dinitrosodiphenyl in benzene solution (10-4 M/l) when it is saturated with O2: 1 - the spectrum of the nitrosocompound before u.v. irradiation; 2 - the spectrum of the same substance after u.v.irradiation during 300 sec. (absorption at 290-300 nm corresponded to one that belonged to 4,4'-dinitrobiphenyl; in addition the reaction product gave positive analytical tests for nitrocompounds).
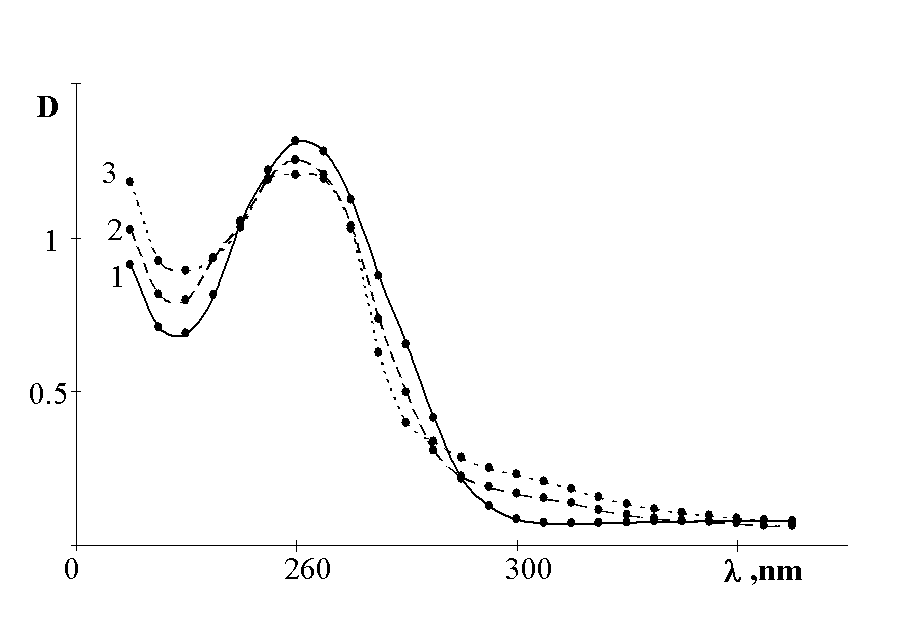
Fig.3. Photooxidation of 4,4'-dinitrosobiphenyl in polysterene films: 1 - before u.v. irradiation; 2 - after u.v. irradiation (during 1 h).
In the result of our report we can conclude that:
(i) Yields of products of the organic azides photooxidation depend on mobility of the reactants in the reaction media. The inhibition of mobility in solid polymer films dimenishes dramatically the yield of nitrocompounds. It means that nitrocompounds formation is second order process analogous (or the same) to reaction 11.
(ii) Nitrosocompounds are the key intermediates of the organic azides photooxidation.
References
1. Abramovitch R.A., Azogu C.I., Sutterland R.G. //Chem.Comm. 1971. P.134-135.
2. Abramovitch R.A., Challand S.R. //J.Chem.Soc.,Chem.Comm.1972.No.16.P.964-966.
3. Singh B., Brinen J.S. //J.Amer.Chem.Soc.1971.V.93.No. P.540-542.
4. Pritchina E.A., Gritsan N.P. // J.Photochem. and Photobiol.A.:Chemistry. 1988.V.43. P.165-182.
5. Sawaki Y., Ishikawa S., Iwamura H. //J.Amer.Chem. Soc. 1987. V.109. No.2. P.584-585.
6. Treuschnikov V.M., Frolova N.V. // Visokomolekularnii Soedinenija.1983.V.25. No.7.P.1400. (in Russ.)
7. Zelentsov S.V. // 4th Conf. on Carbene Chem.Moscow.Nauka. 1987.P.49. (in Russ.)
8. Zelentsov S.V.,Erutov A.V., Ezhevskii A.A., Oleynik A.V.// High Energy Chem. 1997.V.31.P.193.
9. Zelentsov S.V.,Bykova E.A., Ezhevskii A.A., Oleynik A.V.// High Energy Chem. 1997.V.31.P.397.
10. Kuzhnetsov A.N. Spin probe method.Moscow.Nauka.1976. (in Russ.)
11. Wasserman A.M., Kovarskii A.L. Spin label and probe methods in physical chemistry of polymers. Moscow.Nauka.1986. (in Russ.)
12. Rodionov V.A., Rosantsev E.G. Dolgozhivuschii Radikali.Moscow.Nauka.1972. (in Russ.)
13. Bohle D.S., Hansert B., Paulson S.C., Smith B.D.//J.Am.Chem.Soc.1994.V.116. P.7423.