Ultraweak photon emission in human skin cells: Is UV-induced DNA damage involved in intra-and extra-cellular photobiostimulation?
L.A. Applegate1, C. Scaletta1 and H.J. Niggli2*
1Department of Obstetrics
Laboratory of Oxidative Stress and Aging
University Hospital
CH-1011 Lausanne Switzerland
2 BIOFOTON AG, P.O. BOX 28, CH-1731 EPENDES, Switzerland
Tel. / Fax. 0041-264131445
E-mail:biofoton@mail.swissonline.ch
*To whom corrspondence should be sent.
1. Summary
Cyclobutane-type pyrimidine dimers participate as important factors in ultraviolet-induced lethality, mutagenicity and tumorgenicty. Substantial efforts have been made in recent years to understand the induction of pyrimidine photodimers and their repair in human skin cells exposed to low fluences of UV-light. Dimers are efficently induced after UVC and UVB-irradiation, but these photoproducts are even produced in DNA of human skin in vivo after UVA irradiation. By developping a highly sensitive immunohistochemistry dimer detection assay, we confirm that UVA radiation induces substantial amounts of these DNA-changes in the epidermis and this enhanced immunohistochemsitry technique detects them even down to the reticular dermis. A considerable number of these photodimers were also seen in non-irradiated control skin up to 2 cm away from the irradiation site which persist for at least two days postirradiation time. Recent biophysical research has shown the existence of ultraweak photons in biological tissue. UVA is efficently inducing in UVB-irradiated DNA-excision repair deficient XP cells such very weak cell radiation. These results sustain the hypothesis that pyrimidine dimer formation and excision seem be involved in a type of intra- and extracellular photobiostimulation and may be important triggers of UV-induced signal pathways expressing epidermal communication.
2. Introduction
For several decades literature implicates UV-induced DNA damage, especially pyrimidine dimers in deleterious biological events. The relationship between photochemically provoked alterations in DNA by UV-light and carcinogenesis was first illustrated by Cleaver in 1968 [1]. He showed that cells from individuals with Xeroderma pigmentosum, a syndrome in which the patients are extremely prone to sunlight-induced early skin cancer, were defective in DNA repair specifically affecting the excision of pyrimidine dimers. It has been generally accepted that ultraviolet radiation produces the cyclo-butane-type dimer between adjacent pyrimidines on the same DNA strand [2]. However, pyrimidine dimers can also chemically be induced with trimethyl-1,2 dioxetame in DNA in solution in the dark [3]. Pyrimidine dimers alter the biological functions of DNA and they have been shown to be a major cause of lethal and mutagenic [4], tumorigenic [5], and transformational events [6]. More specifically in human and other mammalian skin, pyrimidine dimers have been shown to be involved in many pathways leading to tissue and cell damage including erythema and edema [7,8], keratinocyte damage (sunburn cell formation [9,10], suppressed immunity [11] and tumor formation [12,13].
Recently, beneficial aspects of biological pathways involving pyrimidine dimers have been delineated. Formation of sunburn cells produced via pyrimidine dimers in skin, are important in preventing skin cancer [14]. Sunburn cells are dependent on the gene p53, considered to be the ²guardian-of-the-tissue². This gene prevents mitosis induction prior to accomplished DNA repair and aborts cells damaged beyond repair possibilities. It therefore plays a major role in the prevention of skin tumours.
In another recent study [15] the repair of pyrimidine dimers and/or the accumulation of DNA fragments (topical application of dinucleotides) was shown to be responsible for increased, possibly protective melanogenesis in cultured human skin cells and in mammalian skin. Recent biophysical research has shown the existence of photons in biological tissue. Plant, animal and human cells emit a very weak radiation which can be readily detected with an appropriate photomultiplier system [16]. Although the emission of this radiation is extremely low in mammalian cells, it can be efficiently induced by ultraviolet light. We have reported that postmitotic XP-fibroblasts lose the storing capacity of ultraweak photons which are efficiently trapped in normal cells. Thus, it is evident that there exists an important difference between normal and XP cells and this suggests that there is an effective intracellular mechanism of photon trapping in normal human cells [17]. This type of light-trapping mechanism in DNA could be responsible

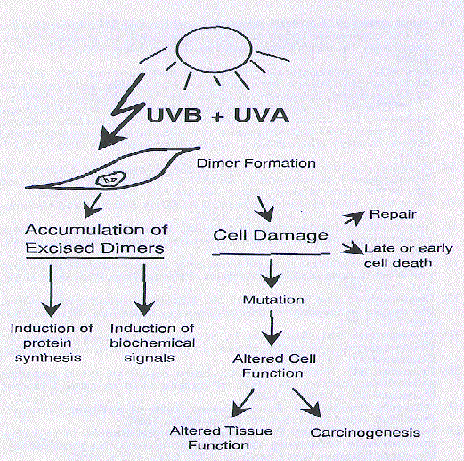
Figure 1. Various agents induce pyrimidine dimer pathways.
for influencing metabolic and cellular events by UV-induced pyrimidine dimers once they are excised as was recently shown for photodimer induced melanogenesis [15].
These recent observations implicating pyrimidine dimers in important signal pathways for possible protection of human skin add new dimensions to the studies where they are shown to trigger mainly deleterious effects as depicted in Figure 1.
We show herein that with sensitive techniques for dimer detection the extent of this DNA modification has perhaps been underestimated in human skin in situ especially after UVA irradiation. The widespread and prolonged induction of this DNA modification suggests as a hypothesis their involvment in a powerful DNA light trapping system in order to convert light energy into biochemical signals. A similar role of light-trapping has been seen for vitamin A in the retinal isomerization reaction found in the light driven photon metabolic process of the eye [18].
3. Material and Methods
3.1. UV-irradiation of human skin and detection of pyrimidine dimers with monoclonal antibodies [19]
For UVA radiation a UVASUN 3000 lamp (Mutzhas, Munich, Germany) was used at a dose rate of 300-600 W/m2 at a distance of 60-90 cm. Irradiation periods were on the average of 10-45 min maximum. The spectral output of the lamp was analyzed with a calibrated Optronic model 742 spectroradiometer (Optronics Laboratories Inc., Orlando, FL, USA). Before and after each experiment radiation fluences were monitored by an International Light Radiometer (IL 1700, calibrated against the spectroradiometer). The emission spectrum was between 340 and 450 nm with a broad peak between 360 and 410 nm and, therefore, this will be termed UVA I herein even though there is a very small amount of visible light present.
The solar simulator used was a 15S single port Solar UV Simulator filtered with Schott WG320/1 mm and UG 11/1 mm filters (290-400 nm) emitting energy similar to that of the overhead sun up to 400 nm (Solar Light Company, Philadelphia, Penn. USA). The dose rate used was between 13-16 W/cm2 and irradiation periods averaged between 2 and 4 min. Radiation exposure (intensity and dose) was continuously controlled by a Dose Control System (Solar Light Company) directly attached to the 15S Solar Simulator which automatically operates a shutter system that closes for a preset dose.
Healthy volunteers between the ages of 24 and 36 with skin type II or III were used for this study under informed consent and the nature of the study, procedures and possible side effects were fully explained (Table I). Separate areas of the buttocks were subjected to increasing doses of either UVA I (25, 50, 75 and 100 J/cm2 or solar simulating radiation (Solar Light 15 S; 30, 40, 50, 60, 70, 80, 100, 120 mJ/cm2). Biopsies of 4 mm were taken under local anesthesia (1% lidocaine) at 24 hours following the UV exposure to ½, 1 and 2 minimal erythema doses (MED) and from adjacent non-irradiated control sites at various distances from the irradiation sites.
Immunohistochemistry for pyrimidine dimers was performed on frozen sections. The frozen sections were subjected to a mild alkaline hydrolysis (0.7 N NaOH for 4 min) followed by digestion with proteinase K (1 mg/ml for 10 min). The sections were then fixed with 4% paraformaldehyde for 30 min at 25° C and washed with PBS 3 times for 10 min each. Thereafter, all incubations were done in a humidified chamber in the dark unless otherwise specified. Tissue sections were incubated with 0.1% phenylhydrazine in PBS for 60 min at 37°C to block endogenous peroxidases and washed 2 times for 5 min each. Non-specific binding was blocked by an incubation for 2 hr at 25°C with a solution of PBS containing 5% fetal calf serum (FCS), 7% normal goat serum (NGS) and 0.1% Triton X 100. They were then incubated overnight at 4° C with H3 monoclonal specific antibodies against thymine dimers (generously offered by Dr. Len Roza, Netherlands) at a 1:50 dilution in PBS containing 5% FCS, 5% NGS and 0.1% Triton X 100. The following morning, tissue sections were washed 3 times for 10 min each in PBS and the sections treated with biotinylated goat anti-mouse at 1:200 in a solution of PBS with 5% FCS, 1% NGS and 0.1% Triton X 100 for 3 hr at 25°C. Tissue sections were washed 4 times for 5 min each in PBS and then treated with Table I- Volunteers for Immunohistochemical detection pyrimidine dimers in UV-Irradiated Skin in situ
Volunteer Age Sex Skin Type
UVA
Radiations:
UVA I
V1 26 M III
V2 36 M II
V3 25 M II
V4 28 M II
V5 28 F II
V6 27 F II
V7 30 M III
Solar UV Simulators:
Solar Light
Co. 15S
V8 25 M II
V9 24 M III
Vectastain ABCâ (Vector, Burlingame, CA) as indicated by the company but diluted in 1% Triton X 100 for 3 hr at 25° C. After this incubation, tissue sections were washed 3 times for 10 min each in PBS and treated with 0.5 mg/ml 3,3’-diaminobenzidine with 0.32 ml 30% H202 added just before an incubation of 1-2 min. The samples were washed for 5 min under running water. They were counterstained with Papanicolaou (Harris’ Hematocylin solution), dehydrated and mounted with Merckoglasâ (Merck, Geneva, Switzerland). All samples were treated at the same time. The antibody stained thymine dimers were visible as a brown coloration.
Control experiments to assure specificity for H3 antibodies were performed with T4-endonuclease V. Tissue sections were treated in 1 M KCL, 20 mM Tris, pH 7, 0.3% Triton X-100 to remove proteins attached to DNA strands. The sections were then incubated with 10 mg/ml of T4 endonuclease V in PBS in order to block dimer antibody binding sites before continuing the immunochemical method for dimer detection.
3.2. Cell culturing and biophotonic measurements.
Skin fibroblasts from a xeroderma pigmentosum patient of complementation group A (XPA), XP12BE (GM05509A) were obtained from the Human Genetic Mutant Cell Respository (Camden,NJ, USA). The cells were grown as monolayers in Dulbecco's modified Eagle's medium (DMEM) with 10% fetal calf serum and 100 U/ml penicillin/streptomycin and UV-irradiated as described elsewhere [17]. The cell suspension in a volume of 10 ml was transferred to a quartz sample tube (2.2x2.2x3.8 cm3). For detection and registration of re-emitted photons the test sample at a cell density of 8x108 cells/ml was kept in a dark chamber in front of a single photon counting device equipped with a EMI 9558 QA photomultiplier tube (diameter of the cathode 48mm, cooled down to -25° C). This high-sensitivity photon counting device is described in detail by Popp and co-workers [17,20,21] and measures photon intensities as low as 10-17 W in the range between 220 and 850 nm. The integral intensity values within given time intervals (4-40ms) were stored and processed by an interfaced
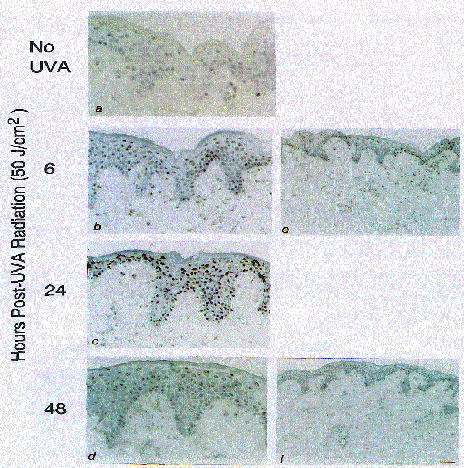
Figure 2. Antibody staining of dimers in human skin in situ following UVA I irradiation.
Dimer antibody staining in previously unirradiated human skin (a) and at, 6 (b), 24 (c), and 48 (d) hours post-UVA radiation (50 J/cm2, 335-450 nm, Mutzhas UVASUN 3000, Germany) (magnification 300x, a-d; 125x, e, f). X 100. The antibody staining for thymine dimers is represented by the brown coloration, the sections were counterstained with Papanicolaou (Harris’ Hematocylin solution). UV-treatment and staining was as described in Material and Methods. In addition, from two of these volunteers, separate areas of buttocks skin were also given 50 J/cm2 and biopsies taken at 0, 3, 6, 24, and 48 hr after UVA I exposure. Biopsies were immediately placed in a beaker with isopentane-2-methyl-butane chilled in liquid
computer. For monochromatic light induction, a monochromater (PTI, Hamburg Germany) was used. Each measuring cycle sat a given wavelength started by irradiating the sample for 30s. The re-emission was measured by two-shutter techniques; one to nitrogen for 2 min. Thereafter, the tissue was transferred to liquid nitrogen for storage until frozen serial sections of 5 mm could be prepared.
stop the irradiation and the other to open a ligh path to the detector as described before [17,20,21].
4. Results and Discussion
4.1. Induction of pyrimidine dimers following UVAI and solar simulating radiation
With avidin-biotin immunohistochemistry techniques using a monoclonal antibody specific for thymine dimers we explored the specific regions of human skin where these dimers localize and to what extent UVAI radiation (320-400 nm) modifies their formation in comparison to solar simulating radiation. Already following sub-erythemal UVB (solar simulating) doses, approximately 10-25% of the epidermal cells showed staining for pyrimidine dimers in any given individual tested as shown in Fig. 2. When comparable erythema doses were given, both UVA I and solar simulating radiation induced a similar quantity of pyrimidine dimers (Table II). Furthermore, the distribution of pyrimidine dimer induction throughout all layers of the epidermis was equivalent. In contrast, UVA radiation was shown to efficiently induce pyrimidine dimers well into the reticular dermis, a process not seen with solar simulating radiation. To assure specificity of the H3 antibodies, experiments were performed on the UV-irradiated tissue sections with T4 endonuclease V. This treatment removed all H3 antibody binding sites on UV-irradiated sections indicating a specificity towards pyrimidine dimers.
The sensitive in situ immunohistochemical assay for thymine dimers has several advantages over the previously performed quantitative measurements. Firstly, one is able to localize the dimers to the different layers of human skin and to assess the extent
Table II- Expression of pyrimidine dimers 24 hr post-UV irradiation of human skin in situ
UVA I Solar Simulator
Pyrimidine dimers
MED: 0 - -
0.5 ND ++
1 +++ +++
2 +++± ++++
Time -
Post-UV: 0 - -
6 ++++ ND
24 +++± ND
48 ++± ND
Multiple stained sections were used to analyze each skin biopsy semi-quantitatively. The staining was interpreted as very strong ++++, strong +++, moderate ++, weak + or no staining -. These observations were noted by two to three investigators independently.
of their presence already before UV treatment. Previous detection methods using the action of Micrococcus luteus UV-endonuclease on isolated DNA, which induces a specific break at dimer sites, does not allow for a precise assessment of initial numbers of lesions. Secondly, dimers in dermal interstitial cells are readily seen with immunohistochemistry, but can not be detected in dermal tissue subjected to DNA extraction procedures (unpublished observation, this laboratory). Unfortunately, quantitation of pyrimidine dimers is rendered difficult due to the tissue processing. As the frozen sections used were subjected to alkaline hydrolysis, there exist obvious holes in the tissue sections where nuclei have disappeared. It was therefore preferable only to semi-quantitate our data.
Many of the UVA induced dimers persist for at least two days. Following UVB exposures, other studies have seen persistence for several days to weeks (Dr. Antony Young, personal communication).
4.2. Persistence of pyrimidine dimers following UVA I radiation
Pyrimidine dimer induction by UVB has been shown to be efficiently repaired in normal human skin cells by excision repair. In one report it was shown that 50% of pyrimidine dimers were excised one hr post-UVB [22], but more recent and extensive studies have shown 50% dimer removal anywhere from 6 to 21 hr following UVB radiation depending on the individual [23]. We have seen in both the dermis and epidermis, the persistance of the induced dimers up to 24 hr post-UVA as shown in Table II. In the 48 hr biopsies, there was decreased antibody staining for thymine dimers in the epidermal keratinocytes and throughout the dermis although a remarkable number of dimers could still be detected 2 days following UV exposure.
4.3. Presence of pyrimidine dimers at various distances from UV-irradiated sites
Investigating pyrimidine dimer induction following UVA irradiation, we found that a considerable number of pyrimidine dimers were also present in the non-UVA-irradiated control skin taken from regions 1-2 cm away from the irradiation sites of the buttock skin as depicted in Fig. 3. The dimers were seen throughout the epidermis; fewer dimers were visible in the rete ridge zone of the dermis. Biopsies analyzed from regions 1-2 cm away from UVB irradiated sites did not show the presence of pyrimidine dimers as seen following UVA radiation (data not shown). At 10 cm from the UVA irradiated sites there were no dimers detected.

Figure 3. Antibody staining of dimers in human skin in situ at various distances from UVA I irradiation sites.
Dimer antibody staining in irradiated human skin (1 minimal erythema dose) and at various distances away from the irradiated site a) 1 cm, b) 2 cm, c) 10 cm. UV-treatment and staining was as described in Material and Methods. Bar is equal to 5 mm.
The presence of pyrimidine dimers at 1-2 cm distances from the actual UVA-irradiated site is an unexpected finding, which can not yet be satisfactorily explained. The most obvious possibility is that of light scattering in the tissue although 2 cm does seem to be extreme for UVA wavelengths. This possiblity would be compatible with the observation that solar simulator (primarily UVB) radiation does not produce the same effect. Another possibility for dimers being induced at distances from the irradiated site is that infrared radiation is capable of inducing the effect. Such a phenomen, with red to infrared was shown in a study by Albrecht-Buehler where cells were capable of communication at distances by a ²rudimentary form of cellular vision² [24]. Another possibility that we can propose for dimers being present at distances from the irradiated site is the induction of pyrimidine dimers indirectly by the secretion of a factor or cytokine. If a chemical such as trimethyl-1,2 dioxetame can induce pyrimidine dimers in DNA in vitro [3] , it can not be completely ruled out that photsensibilsating substances may also permit the same reaction to occur in vivo. In this respect in the UVB, dimer yields are influenced by the irradiation temperature [25] , and the nucleosomal linker DNA is enriched in dimer content by a factor of 2-4 relative to bulk DNA [26] . In contrast, neither temperature [27] nor nucleosomal structure affect dimerization in the UVC-region.
4.4. Pyrimidine dimers as photon storing vehicels in DNA in order to convert light energy into biochemical signals
A new hypothesis is proposed on the basis that light itself is responsible for this effect. (see scheme 1) In this respect, spontaneous ultraweak photon emission (PE) has been extensively described in yeast, plant and animal cells [16,28-33]. In a recent report, experiments with cultured human cells in which normal and DNA-excision-repair-deficient XP cells were UV-irradiated in medium and balanced salt solution (BSS) were assessed for ultraweak photon emission [17]. There was evidence of induced-photon emission from normal cells in BSS but clear evidence of a UV fluence-dependent emission in XP cells in medium and in BSS. Overall, these results revealed an important
Scheme 1. Pyrimidine dimers as a powerful light trapping system in DNA in order to convert light energy into biochemical signals .
Proposed mechanism of pyrimidine dimers as a powerful light trapping system in DNA in order to convert light energy into biochemical signals in comparison to the mechanism of the light-driven photoisomerization of the chromophore retinal discovered 25 years ago in bacteriorhodopsin.
![]()
![]()
ò ò
Rhodopsin DNA
ò ò
activated Photodimerization of
Rhodopsin DNA
ò òexcision repair
Transducin G-Protein
GTP/ATP-complex GTP-complex
ò ò
Activation of phosphodiesterase Activation of adenylate cyclase
difference between normal and XP cells and it was proposed that XP cells are unable to store ultraweak photons which are efficently trapped in normal cells and perhaps used to regulate metabolic activity [17]. In the same study, we found in defined stages of the fibroblast differentiation system, which has been recently described in detail by Bayreuther and co-workers [34,35], that UV-light elevates photon emission in MMC-induced postmitotic XP-fibroblasts at least by a factor of 2 compared to mitotic XP-cells. As depicted in Fig. 4, these ultraweak photons in human skin fibroblasts from a xeroderma pigmentosum patient of complementation group A (GM05509A obtained from the Human Genetic Mutant Cell Respository (Camden, NJ, USA)) are efficiently induced with light in the UVAI region (360-420 nm). As shown by Kay and co-workers [36] circadian oscillators are present throughout the body of drosophila which are switched on most probably by light. This study is conforming the recent results of Cohen and Popp [37] which found specific biological rhythms for the ultraweak photon emission in the hands and forehad of the human body. In this respect Sancar discovered that plant and mammalina cells have blue-light sensitive proteins that have nothing to do with DNA repair [38] . It is very likely, however, that not only proteins but also nucleic acids, as shown in Scheme 1, act as chromophores in the genetic material. An important part may be involved via the UV light-induced photochemical reaction of dimer formation in a storage process of light energy into DNA with subsequent activation of biochemical reactions, a similiar mechanism as the light-driven photoisomerization of the chromophore retinal discovered 25 years ago in bacteriorhodopsin [18] and subsequently confirmed in the visualisation pathway of the eye. The last hypothesis would support that dimer formation and excision could be an expression of epidermal communication and not only a sign of UV damage. As recently shown by Gilchrest and co-workers [15], UV-induced pyrimidine photodimers are stimulating melanogenesis confirming therefore that photodimers initiate biochemical processes and serve as molecular signals. In conclusion, our data show experimental evidence that the genetic material is involved in the ultraweak photon emission process and therefore in intra- and extracellular biocommunication. Furthermore, our results imply that the complex mechanism of UV-induced DNA damage and repair may be a powerful light trapping system in DNA in order to convert light energy into biochemical signals and are therefore involved in a intra- and extracellular photobiostimulation.
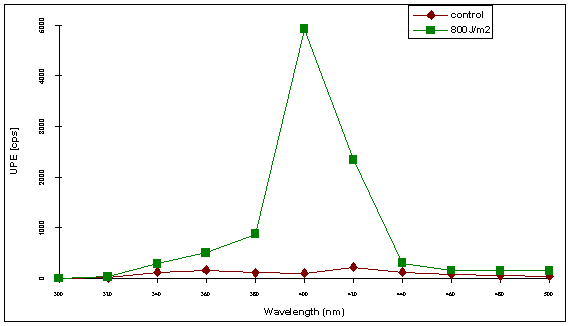
Figure 4. Monochromatic light induced photon emission in repair deficient XPA human skin fibroblasts.
Spectral light induced ultraweak photon emission 6 h after UV-irradiation of 8 million postmitoic XPA-cells with 800 J/m2 UVB in comparision to unirradiated control postmitoic XPA-cells. Initial counts of photoinduced ultraweak photon emission were registered in the first 10 milliseconds after illumination with monochromatic light during 30 s of repair deficient Xeroderma Pigmentosum human skin fibroblasts (GM05509 A). Background values obtained with medium are subtracted. Error bars shown represent standard deviations of two independent experiments with different cells.
5. References
1. Cleaver, J.E: Defective repair of DNA in xeroderma pigmentosum, Nature 218 (1968) 652.- 656.
2. Setlow, R. B: Cyclobutane-type pyrimdine dimers in polynucleotides, Science 153 (1966) 379- 386.
3. Lamola, A.A.: Production of pyrimidne dimers in the dark. Biochem. Biophys. Res. Commun. 43 (1971), 893-898
4. Harm, H: Repair of UV-irradiated biological systems: photoreactivation. In Photochemistry and Photobiology of Nucleic Acids , ed Wang, Y. (Academic, New York, 1976) pp 219-263.
5. Hart, R. W., Setlow, R. B. and A. D. Woodhead: Evidence that pyrimidine dimers in DNA give rise to tumors, Proc. Natl. Acad. Sci(USA) 75 (1977), 5574-5578.
6. Sutherland, B. M., Cimino, J. S., Delihas, N., Shih, A. G. and R. P. Oliver: Ultraviolet light- induced transformation of human cells to anchorage-independent growth, Cancer Res. 40 (1985), 2409-2411 .
7. Ley, R. D: Photoreactivation of UV-induced pyrimdine dimers and erythema in the marsupial Monodelphis domestica, Proc. Natl. Acad. Sci. (USA) 82(1985), 2409-2411 .
8 Van Wheelden, H: Lasers in Photomedicine and Photobiology (eds Patresi, R., Sacci, C. A.) (Springer Series in Optical Sciences, Berlin,1980) pp 129-133.
9. Ley, R. D. and L. A. Applegate : Ultraviolet radiation-induced histopathologic changes in the
skin of the marsupial Monodelphis domestica. II. Quantitative studies of the photoreactivation of induced hyperplasia and sunburn cell formation. J. Invest. Dermatol. 85 (1985), 365-367.
10. Ley, R. D. and L. A. Applegate: Loss of photoreversibility of sunbrun cell induction in Monodelphs domestica. Photochem. Photobiol. 46 (1987), 315-317.
11. Applegate, L. A., Ley, R. D., Alcalay, J. and M. L. Kripke: Identification of the molecular target for the suppression of contact hypersensitivity by ultraviolet radiation. J. Exp. Med. 170 (1989), 1117-1131.
12 Ley, R. D., Applegate, L. A., Fry, R.J. and A. B. Sanchez : Photoreactivation of ultraviolet radiation induced skin and eye tumors of Monodelphs domestica. Cancer Res. 51 (1991), 6539- 6542.
13. VandeBerg, J. L., Williams-Blangero, S., Hubbard, G. B., Ley, R. D. and E. S. Robinson : Genetic analysis of ultraviolet radiation-induced skin hyperplasia and neoplasia in a laboratory marsupial model (Monodelphs domestica).Arch. Derm. Res. 286 (1994), 12-17.
14. Ziegler, A., Jonason, A. S., Leffell, D. J., Simon, J. A., Sharma, H. W., Kimmelman, J., Remington, L., Jacks, T. and D. E. Brash : Sunburn and p53 in the onset of skin cancer. Nature 372( 1994), 773-776.
15. Eller, M.S., Yaar, M. and B.A. Gilchrest: DNA damage and melanogenesis, Nature 372 (1994)
413 -414.
16. Niggli H.J., The cell nucleus of cultured melanoma cells as a source of ultraweak photon emission, Naturwissenschaften 83 (1996) 41-44.
17. Niggli, H.J.: Artificial sunlight irradiation induces ultraweak photon emission in human skin fibroblasts, J. Photochem. Photobiol. B: Biol.,18 (1993) 281-285
18. Oesterhelt, D. and W. Stoeckenius: Rhodopsin-like protein from the purple membrane of Halobacterium halobium. Nature 233 (1971) 149-151.
19. Applegate, L. A. , Scaletta, C. Panizzon, R. Niggli, H. and E. Frenk: In vivo induction of pyrimdine dimers in human skin by UVA radiation: Initiation of cell damage and/or intercellular communication. Intern. J. of Molecular Med. 3 (1999) 467-472.
20. Popp, F.A., Li , K.H. and Q. Gu, Recent advances in Biophoton Research and its Applications (World Scientific, Singapore,1992).
21. Chang, J.J., Fisch, J. and F. A. Popp: Biophotons (Kluwer Academic Publishers, Dordrecht, 1999).
22. D' Ambrosio S.M., Slazinski, L., Whetstone, J.W. and E. Lowney: Photorepair of pyrimdine dimers in DNA in human skin in vivo.J. Invest. Dermatol. 77 (1981) 311-313.
23. Niggli, H.J. and P.A. Cerutti : Cyclobutane-type pyrimidine photodimer formation and excision
in human skin fibroblasts after irradiation with 313-nm ultraviolet light. Biochemistry 22 (1983)
1290-1295.
24. Albrecht-Buehler, G.: Rudimentary form of cellualr 'vision'. Proc. Natl. Acad. Sci. USA 89
(1992)
8288-8292.
25. Niggli, H.J. and P.A. Cerutti : Temperature dependence of induction of cyclobutane-type pyrimidine photodimers in human skin fibroblasts after irradiation with 313-nm ultraviolet light. Photochem. Photobiol. 37 (1983) 467-469.
26. Niggli, H.J. and P.A. Cerutti : Nucleosomal distribution of thymine photodimers following far and near-ultraviolet irradiation. Biochem. Biophy. Res. Commun.105 (1983) 1215-1223.
27. Niggli, H.J.: A new sensitive assay for detecting thymine containing pyrimdine dimers using HPLC. In Proceedings of the first Interantional meeting on cosmetic dermatology in Rome, Italy, March 7-9, 1985 eds. Morganti, P. and W. Montagna (Ediemme, Rome, 1986) pp 335-342.
28 Multi-author review on Biophoton emission: Chwirot,W.B. , G. Cilento, A.A. Gurwitsch, H. Inaba, W. Nagl, F.A. Popp, K.H. Li, W.P. Mei, M. Galle, R. Neurohr, J. Slawinski, R.V. Van Wijk and D.H.J. Schamhart, Experientia 44 (1988) 543 -600.
29. Niggli, H.J. : Ultraweak photons emitted by cells: biophotons, J. Photochem. Photobiol. B: Biol. 14 (1992a) 144 -146.
30. Multi-author review on biophoton emission, stress and disease: Tilbury, H.J. ,J. Slawinski, A. Ezzahir, M. Godlewski, T. Kwiecinska, Z. Rajfur, D. Sitko, D. Wienzuchowska, B. Kochel, Q. Gu, F.A. Popp, E.M. Lilius, P. Marnila, R. Van Wijk and J.M. Van Aken, Experientia 48 (1992) 1029 -1102.
31. Scholz, W., U. Staszkiewicz, F.A. Popp and W. Nagl: Light-stimulated ultra-weak photon re- emission of human amnion and wish cells, Cell Biophys. 13 (1988) 55-63.
32. Van Wijk, R.V., W.P. Mei, F.A. Popp and H. Van Aken: Light-induced photon emission by mammalian cells, Photochem. Photochem.B: Biol. 18 (1993) 75-79.
33. Niggli, H.J. :Erfahrungsheilkunde48 (1999) in press.
34. Bayreuther, K., H.P. Rodemann, R. Hommel, K. Dittman , M. ,Albiez and P.I. Francz: Human skin fibroblasts in vitro diferentiate along a terminal cell lineage, Proc. Natl. Acad. Sci. U.S.A. 85 (1988) 5112-5116.
35. Niggli, H.J., K. Bayreuther, H.P. Rodemann, R. Röthlisberger and P.I. Francz:Mitomycin C- induced postmitotic fibroblasts retain the capacity to repair pyrimidine photodimers formed after UV-irradiation, Mut. Res. 219 (1989) 231-240.
36. Plautz, J.D., Kaneko, M., Hall, J.C. and S.A. Kay: : Independent photoreceptive circadian clocks throughout Drosophila, Science 278 (1997) 1632 -1635.
37. Cohen, S. and F.A. Popp: Biophoton emission of the human body, Photochem. Photochem.B: Biol.40 (1997) 187-189.
38. Miyamato, Y. and A. Sancar: Vitamine B2-based blue-light photoreceptors in the retinohypothalamic tract as the photoactive pigments for setting the circadian clock in mammals, Proc. Natl. Acad. Sci. U.S.A. 95 (1999) 6097-6102 .
Acknowledgements :
The authors wish to thank Prof. E. Frenk for the biopsies of the volunteers and Simone Pradervand and Franca Labidi for help with some of the histological sectioning. This study was supported by grants from the Swiss National Fund (LAA, 3100-049120.96/1), the Research Swiss Against Cancer (KFS 695-7-1998) and Cosmital SA (Switzerland) a Company of Wella (Germany).