

PALACKY UNIVERSITY OLOMOUC, CZECH REPUBLIC
THE CHEMIEXCITATION OF THE PHOTOSENSITIZERS
Martina Bancirova
1; Hana Kolarova1; Rene Lenobel3; Jitka Frebortova1, Jarmila Medkova3, Jan Lasovsky21
Centre of Molecular Biology and Medicine, Palacky University, Olomouc 783 71,2
Department of Inorganic and Physical Chemistry, Palacky University, Olomouc 771 46,3
Department of Botany, Palacky University, Olomouc 783 71, Czech RepublicE-mail: bancir@aix.upol.cz
![]()
Abstract
Photodynamic therapy (PDT) is a treatment that is used for the destruction of certain tumors. The treatment is performed with the photosensitizers that generate reactive oxygen species (ROS) in the presence of light and oxygen. The absence of light in many regions of the body may preclude the use of the photosensitizers as therapeutic compounds for PDT. It is known that the chemiluminescent oxidation of luciferin is able to generate a sufficiently intense and long-live emission to induce antiviral activity of hypericin.
In our chemiluminescent system we assumed a formation of the micellar complex of electron transfer between hydrogen peroxide and dyes and formation of their excited states. It has been found that the chemiexcited photosensitizers could produce photodynamic damage on Gram-negative and Gram-positive bacteria and in cellular cultures.
Introduction
Photodynamic therapy (PDT)
[1] is a treatment that is used for the destruction of certain tumors. PDT involves the combination of visible light and an organic dye - photosensitizer. Each factor is harmless by itself, but when is combined with oxygen, they can produce lethal cytotoxic agents that can inactivate tumor cells. Upon illumination, the photosensitizer is excited from the ground state to the first excited single state, followed by conversion to the triplet state via intersystem crossing. The excited triplet state can react in two ways. A Type I mechanism involves hydrogen-atom abstraction or electron-transfer reactions between the excited state of the photosensitizer and the substrate to yield free radicals and radicals ions. A Type II results from an energy transfer between the excited triplet state of the photosensitizer and the ground state molecular oxygen generating the first excited state of oxygen, singlet oxygen. The absence of light in many regions of the body may preclude the use of the photosensitizers as the therapeutic compounds for PDT. It has been found that the chemiluminescent oxidation of luciferin is able to generate a sufficiently intense and long-lived emission to induce antiviral activity of hypericin [2], a well-known photosensitizer.Weak chemiluminescence accompanies the catalytic decomposition of hydrogen peroxide. It is possible to enhance this light emission and therefore also the population of the excited states by means of oxidizable substrates with the high quantum yield of excitation (energizers e.g. phthalhydrazide (PH) and luminol (LU)
[3,4]. It is possible to transfer their excitation energy to the suitable acceptor (e.g. photosensitizer).
Materials and Methods
Instrumentation:
Luminometer BioOrbit 1250 (Finland), Luminoskan and Fluoroskan Ascent (Labsystems, Finland) and inversion fluorescent microscope Olympus IX 70, Laser LASOTRONIC MED-130 (Germany).Reagents
: phthalocyanines (ClAlPcS2, ZnPcS2, CuPcS4 (CU), NiPcS4 (NI)), phthalhydrazide (PH), protoporphyrin IX (PP), methylene blue (MB), eosin B (EB), CuSO4, H2O2 (Sigma-Aldrich, Germany), hypericin (HY), hypocrellin A (HA), luminol (LU), calcein AM (Molecular Probes, USA), all media for MIC determination were obtained from HiMedia Laboratories (Bombay, India).Bacterial strains
: Escherichia coli 3954, 3988 and 4225, Pseudomonas aeruginosa 3955, Staphylococcus aureus 3953 and 4223, and Enterococcus faecalis 4224 (Czech Collection of Microorganisms, Brno).Cell cultures:
NIH3T3 (mouse fibroblast), MCF7 (human breast adenocarcinoma), HOS (osteosarcom) (Masaryk Memorial Cancer Institute, Brno-Czech Republic)Methods
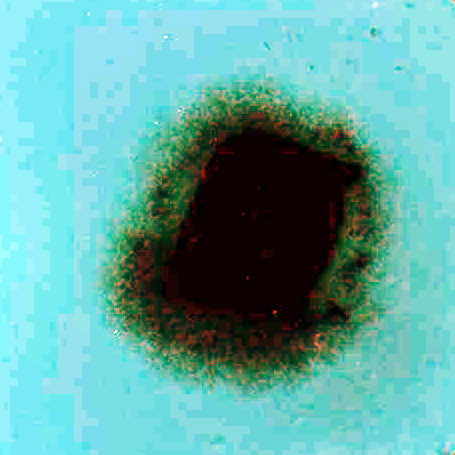
The migration activity of splenocytes [5] derived from rabbit spleen fragments was determined as follows. Aseptically excised rabbit (Chinchilla) spleen was cut on a sterile paraffin plate into pieces 1 mm in diameter. These fragments were washed in minimal essential medium (MEM). Increasing concentrations of the additives (photosensitizers, phthalhydrazide, CuSO4, H2O2) in MEM with 10% fetal calf serum (FCS) were pipetted in volumes of 0,2 mL into wells (plates 8x12, flat bottom). Each combination of the additives was placed into four wells; one fragment was transferred into each well. The fragments in cultivation medium only were used as a control. After 24 hours of cultivation at 37oC in 5% CO2, the migration zones of the spleen cells were measured [5]. The results are presented as the migration index (MI) - the ratio between the experimental migration zone (Fig.I.B.) to the control zone (Fig.I.A.). Compounds were tested in two independent experiments in triplicate.
Figure I. Fragments of the rabbit's spleen splenocytes
A. …………………………………………………………………..B.
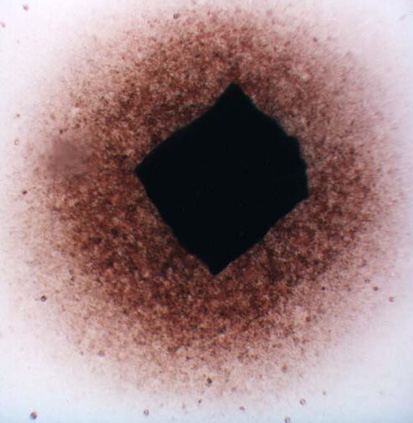 ………………………………………..
………………………………………..
Minimal inhibitory concentration (MIC) [6] Antimicrobial activity was tested on the standard strains of Escherichia coli 3954, 3988 and 4225, Pseudomonas aeruginosa 3955, Staphylococcus aureus 3953 and 4223, and Enterococcus faecalis 4224 (Czech Collection of Microorganisms, Brno). Bacteria were first precultured 2 h at 37°C in 5 mL of Mueller-Hinton broth, then diluted with sterile distilled water in a 1:10 ratio, and inoculated into the wells (sterile plates 8x12, flat bottom) containing tested compound in cultivating medium. Gram-positive bacteria were cultivated in a medium containing 37g Brain Heart Infusion in 1 L of distilled water. Medium for Gram-negative bacteria consisted of 5 g Protose-BE, 17,5 g Casein Acid Hydrolysate and 0,6 g Na2CO3 in 1 L of distilled water. Minimal inhibitory concentrations (MICs) were determined by broth microdilution method. The stock solutions of tested compounds were prepared in distilled water. The plates were incubated 18 h at 37°C and MIC was determined as the lowest concentration of the tested compounds at which bacterial growth was not observed (content of well stays without turbidity). Compounds were tested in concentrations in geometrical dilution steps, in three independent experiments in triplicate.
Test of viability
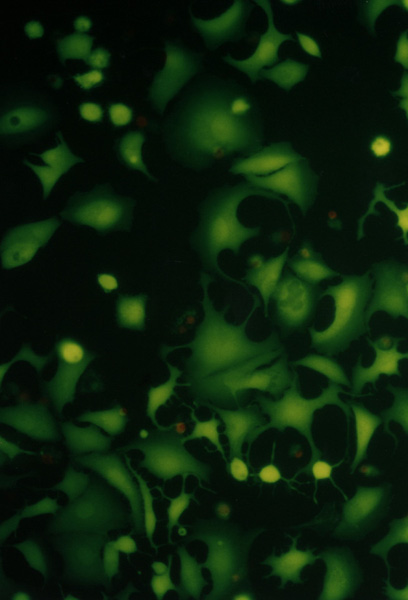
[7] NIH3T3 (mouse fibroblast), MCF7 (human breast adenocarcinoma) and HOS (osteosarcom) were used as a standard testing system. Twice washed trypsinized cells were divided in the amount of 104 to each well and filled in DMEM with 10% FCS in a total volume of 80 µL. After 24 hours of cultivation at 37°C in 5% CO2 the photosensitizer with chemiluminescent system (20 µL) was added. The total volume of 100 µL (cells with additives) were cultivated for 24 hours. For identification of live and dead cells the fluorescent probes calcein AM was used. For the elimination of the influence of fluorescence from the photosensitizer the testing wells were aspirated, washed with 100 µL PBS and filled with 100 µL PBS and 100 µL calcein AM. The results were proved by fluorometric measurement with Fluoroskan Ascent. Morphological changes of the cells during cultivation have been observed in an Olympus IX 70 inversion fluorescent microscope. Compounds were tested in three independent experiments in quadruple.Figure II. NIH3T3, HOS, MCF7
Cell cultures (NIH3T3, HOS, MCF7) observed in Olympus IX 70 fluorescent microscope, magnification 300.
…………… ………….…
………….…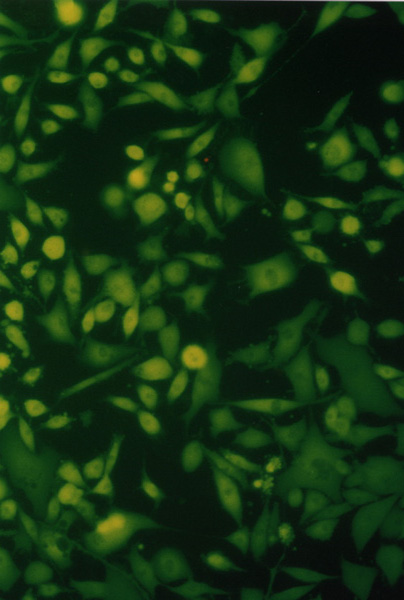 ………….….
………….….
Results and Discussion
The chemiexcitation of the xanthene dyes and photosensitizers (e.g. hypericin, phthalocyanines) induced by the hydrogen peroxide decomposition and autooxidation by dissolved oxygen in the micellar solutions of the cetyltrimethylammonium cation was studied in our previous work
[4]. We subscribe to the CIEEL theory a formation of the micellar complex of electron transfer between hydrogen peroxide and dyes and formation of their excited states in the chemiluminescent system. Observed facts can be phenomenologically explained using the idea of the formation of micellar complexes of dyes according to Poisson Distribution Model (PDM). Both chemiluminescent intensity and chemiexcitation rate depend on the rate of hydrogen peroxide decomposition. The chemiexcitation does not depend on the structural type of dye. The final step is peroxide decomposition, back electron transfer to dyes and formation of their excited states. The energy transfer by energy pooling is only the minority way. The goal of this work was to find the efficiency of the chemiexcitation of the photodynamic effect on the biological material.The migration activity of splenocytes
As a first
we confirmed that the photosensitizers chemiexcited by phthalhydrazide can produce the photodynamic damage on the rabbit’s spleen (test of vitality of the migration activity of splenocytes derived from rabbit's spleen fragments).This test turned up to be the simple, versatile and reliable for photodynamic effect in connection with sensitized chemiluminescence. For the dependence on the elementary concentrations of the photosensitizer, compounds of the chemiluminescent system and on their combinations, the data display the changes of the migration index. The changes can be neglected for the compounds of the chemiluminescent system but they are significant for the individual photosensitizer and its combination with the chemiluminescent compounds. Dependencies of MI on the photosensitizer concentration, eventually on the photosensitizer concentration at the presence of compounds of the chemiluminescent system, complied with simple Langmuir isotherms. For this reason we were able to assume that photosensitizer is adsorbed in the biological membrane and the presented data indicate that observed lethality is a result of photodynamic effect.
Tables I. and II. show review c50 (concentration calculated according to Langmuir isotherms, at which MI decrease in 50%) for the chemiexcited photodynamic effect.Table I. The migration activity (without irradiation by laser)
c50 for migration activity of splenocytes of the rabbit's splenocytes in the presence of the photosensitizer without additives and in the presence of the photosensitizer in combination of the compounds of the chemiluminescent system
|
PH |
CuSO4 (1.10-8M) H2O2 (1.10-6 M) |
ClAlPcS2 |
ZnPcS2 |
c50 Photosensitizer (mg/mL) |
Determination Coefficient |
|
j |
j |
X |
n |
0,056 |
0,8000 |
|
j |
X |
X |
j |
0,040 |
0,8366 |
|
8.10-6 M |
X |
X |
j |
0,027 |
0,9486 |
|
j |
j |
J |
X |
0,042 |
0,6708 |
|
j |
X |
j |
X |
0,027 |
0,8944 |
|
8.10-6 M |
X |
j |
X |
0,011 |
0,9434 |
Table II. The migration activity (after irradiation by laser)
c50 for migration activity of splenocytes of the rabbit's splenocytes in the presence of the photosensitizer without additives and in the presence of the photosensitizer in combination of the compounds of the chemiluminescent system after irradiation by laser.
|
PH |
CuSO4 (1.10-8 M) H2O2 (1.10-6 M) |
ClAlPcS2 |
c50 Photosensitizer (mg/mL) |
Determination Coefficient |
|
j |
j |
X |
0,023 |
0,8367 |
|
8.10-6 M |
X |
X |
0,007 |
0,9591 |
Figure III. Effect of chemiexcited phthalocyanines on the migration activity
The figure shows an example of the dependence of the MI on the concentration of the photosensitizer (ClAlPcS2 and ZnPcS2) [phthalocyanine] and on the concentration of the photosensitizer with the addition of the phthalhydrazide [phthalocyanine + PH] at the presence of CuSO4 and H2O2 (CuSO4 -1.10-8 M and H2O2 -1.10-6 M).
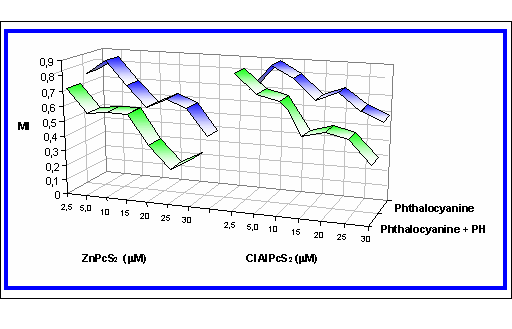
These results confirm the possibility to increase the photosensitizer efficiency by the chemiexcitation.
Minimal inhibitory concentration (MIC)
Bacterial resistance against antibiotic treatment is becoming an increasing problem in medicine. Therefore methods to destroy microorganisms by other means are being investigated, one of which is photodynamic therapy. It has already been shown that a variety of Gram-positive and Gram-negative bacteria can be killed in vitro by PDT
[8]. Therefore we have studied the chemiexcitation of photosensitizer (phthalhydrazide (2.10-4 M) CuSO4 (1.10-6 M) and H2O2 (2,5.10-5 M)) on Gram-positive and Gram-negative bacteria. The minimum inhibitory concentration has been found for the presence of the photosensitizers and for the photosensitizer in the presence of the chemiluminescent system: Gram-positive bacteria Enterococcus faecalis: 0,545 mg/mL ClAlPcS2, 0,327 mg/mL ClAlPcS2 in the presence of PH, and Staphylococcus aureus: 0,545 mg/mL ClAlPcS2, 0,188 mg/mL ClAlPcS2 in the presence of PH, 1,114 mg/mL ZnPcS2, 0,265 mg/mL ZnPcS2 in the presence of PH, Gram-negative bacteria Pseudomonas aeruginosa: 0,667 mg/mL ClAlPcS2, 0,333 mg/mL ClAlPcS2 in the presence of PH more than 1,2 mg/mL ZnPcS2, 0,133 mg/mL ZnPcS2 in the presence of PH, Escherichia coli: more than 1,2 mg/mL ClAlPcS2, 0,375 mg/mL ClAlPcS2 in the presence of PH, more than 1,2 mg/mL ZnPcS2, 0,265 mg/mL ZnPcS2 in the presence of PH.
Table III. Minimal inhibitory concentration of the photosensitizers on Gram-positive bacteria
* - the concentration used was up to 1,5 mg/mL. Number in the brackets shows the concentration of phthalocyanines (mg/mL) in the presence of phthalhydrazide but without CuSO4 and H2O2.
|
j |
ClAlPcS2(mg/mL) |
ZnPcS2 (mg/mL) |
ClAlPcS2(mg/mL) Chemiexcited |
ZnPcS2 (mg/mL) Chemiexcited |
|
Enterococcus faecalis 4224 |
0,545 |
- * |
0,327 (0,210) |
- * |
|
Staphylococcus aureus 3953 |
0,506 |
1,114 |
0,188 (0,210) |
0,310 (-) |
|
Staphylococcus aureus 4223 |
0,506 |
- * |
0,375 (0,210) |
0,265 (-) |
Table IV. Minimal inhibitory concentration of the photosensitizers on Gram-negative bacteria
* - the concentration used was up to 1,5 mg/mL. Number in the brackets shows the concentration of phthalocyanines (mg/mL) in the presence of phthalhydrazide but without CuSO4 and H2O2.
|
j |
ClAlPcS2(mg/mL) |
ZnPcS2 (mg/mL) |
ClAlPcS2(mg/mL) Chemiexcited |
ZnPcS2 (mg/mL) Chemiexcited |
|
Pseudomonas aeruginosa 3955 |
0,667 |
- * |
0,333 (0,667) |
0,265 (-) |
|
Escherichia coli 3954 |
- * |
- * |
0,375 (-) |
0,265 (-) |
|
Escherichia coli 3988 |
- * |
- * |
0,375 (-) |
0,265 (-) |
|
Escherichia coli 4225 |
- * |
- * |
0,375 (-) |
0,265 (-) |
It has been found that the chemiexcitation increases the photodynamic damage on Gram-positive and Gram-negative bacteria:
Test of viability
In the last part we have focused on the chemiexcited photodynamic damage in the different cellular cultures. Viability of cells was determined by means of the molecular probes for fluorescence microscopy (calcein AM).
The chemiexcited photodynamic damage was studied in the following cell cultures: NIH3T3 (mouse fibroblast) and MCF7 (human breast adenocarcinoma). The photosensitizers were chemiexcited by phthalhydrazide or by luminol. The concentration used were: phthalhydrazide 5.10-6 M, luminol 5.10-6 M, CuSO4 5.10-6 M, H2O2 1.10-6 M.
Table V. shows the cytotoxicity (IC50 for NIH3T3) of the photosensitizer
(without the chemiexcitation) and of the photosensitizer chemiexcited by phthalhydrazide or by luminol.
Table V. Cytotoxicity in NIH3T3 cells.
|
. |
Photosensitizer |
Photosensitizer chemiexcited by phthalhydrazide |
Photosensitizer chemiexcited by luminol |
|
Protoporphyrin IX |
0,3870 mg/mL |
0,3500 mg/mL |
0,3500 mg/mL |
|
Hypericin |
0,0123 mg/mL |
0,0093 mg/mL |
0,0093 mg/mL |
|
Hypocrellin A |
0,0063 mg/mL |
0,0016 mg/mL |
0,0016 mg/mL |
|
CuPcS4 |
0,7000 mg/mL |
0,3500 mg/mL |
0,3500 mg/mL |
|
NiPcS4 |
0,2970 mg/mL |
0,0110 mg/mL |
0,0110 mg/mL |
|
Methylene Blue |
0,0220 mg/mL |
0,0110 mg/mL |
0,0110 mg/mL |
|
Eosin B |
0,2640 mg/mL |
0,3500 mg/mL |
0,3500 mg/mL |
Table VI. shows the cytotoxicity (IC50 for MCF7) of the photosensitizer
(without the chemiexcitation) and of the photosensitizer chemiexcited by phthalhydrazide or by luminol.
Table VI. Cytotoxicity in MCF7 cells.
|
. |
Photosensitizer |
Photosensitizer chemiexcited by phthalhydrazide |
Photosensitizer chemiexcited by luminol |
|
Protoporphyrin IX |
0,7000 mg/mL |
0,0420 mg/mL |
0,0310 mg/mL |
|
Hypericin |
0,0224 mg/mL |
0,0300 mg/mL |
0,0190 mg/mL |
|
Hypocrellin A |
0,0063 mg/mL |
0,0016 mg/mL |
0,0016 mg/mL |
|
CuPcS4 |
0,7000 mg/mL |
0,3500 mg/mL |
0,3500 mg/mL |
|
NiPcS4 |
0,3200 mg/mL |
0,0360 mg/mL |
0,0660 mg/mL |
|
Methylene Blue |
0,0220 mg/mL |
0,0110 mg/mL |
0,0110 mg/mL |
|
Eosin B |
0,0910 mg/mL |
0,3500 mg/mL |
0,3500 mg/mL |
Figure IV. The comparison of the chemiexcitation of the photodynamic effect by phthalhydrazide and by luminol in the MCF7 cellular culture
The figure shows an example of the influence of energizer (LU-luminol, PH-phthalhydrazide, NI-NiPcS4, PP-Protoporphyrin IX, HY-hypericin, HA-hypocrellin A).
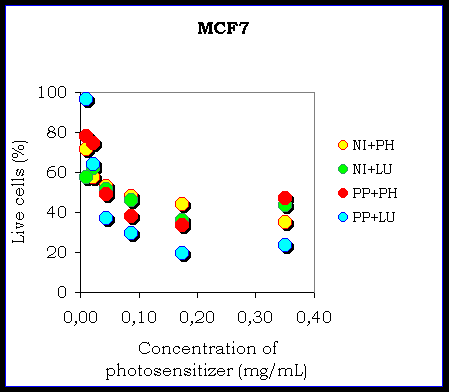
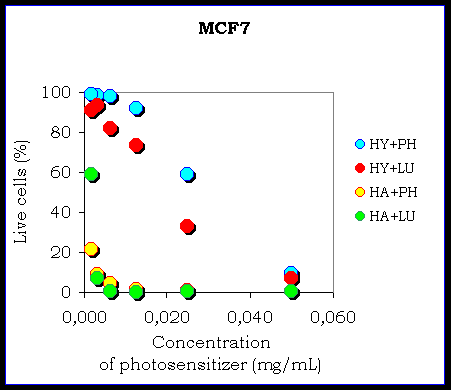
The results confirm the possibility of the chemiexcitation of the photosensitizer in the cellular cultures. There is a different efficiency of the chemiexcitation. It depends on the type of the photosensitizer and on the type of the cellular culture because there is a different ability of the photosensitizers to remain in the cells and also the different ability of the photosensitizers to produce reactive oxygen species. The influence of the energizer (phthalhydrazide or luminol) seems to be negligible.
Conclusion
The chemiexcitation of the photosensitizers induced by the hydrogen peroxide decomposition and autooxidation by dissolved oxygen in the micellar solutions of the cetyltrimethylammonium cation was studied in our previous work. The chemiexcitation of the photosensitizers induced by the hydrogen peroxide decomposition on the biological material was studied. We confirmed possibility of the photodynamic damage after the chemiexcitation on the rabbit’s spleen (test of vitality of the migration activity of splenocytes derived from rabbit spleen fragments), on Gram-positive and Gram-negative bacteria and also in the cellular cultures. The efficiency of the chemiexcitation depends on the type of the photosensitizer and on the type of the cellular culture, on their mutual combination. The influence of the energizer (phthalhydrazide or luminol) seems to be negligible (we haven’t found considerable differences).
Acknowledgments
This research was supported by the grant from Ministry of Education No. VS 96 154, post-doc grant No. 203/98/P257 of the Grant Agency of the Czech Republic and by the "KONTAKT" No. 17/26, programme based on the agreement between the governments of Italy and the Czech Republic.
References:
http://www.photobiology.com/photo99/contrib/kolarova/index.htm