| Site home page | Conference home page | Discussion |
4,5’,8-trimethylpsoralen induces numbers of DNA-protein cross-links
1 Department of Pharmaceutical Sciences of Padova University; Centro di Studio sulla Chimica del Farmaco e dei Prodotti Biologicamente Attivi del C.N.R.; Padova, Italy. 2 Department of Genetics of Carol Davilà University, Bucharest, Romania.
Studying the damage induced into DNA by furocoumarin sensitization and especially covalent DNA-protein cross-links (DPC), we have observed that an angelicin isoster, namely 1,4,6,8-tetramethyl-2H-furo[2,3-h]quinolin-2-one (FQ), can selectively induce DPC when used by means of the well-known method called double irradiation [Bordin et al., 1996; 2000]. This method was introduced studying furocoumarin inter-strands cross-links (ISC), which are formed by a biphotonic reaction [Ben-Hur and Elkind, 1973]. According to this protocol, after a first irradiation step, during which the sensitizer mainly induces DNA monoadducts (MA), the unbound furocoumarin molecules are washed out and the system irradiated again. MA can further react with another DNA base, yielding biadducts, i.e., ISC. Therefore, using the double irradiation protocol, furocoumarins can induce ISC but no additional MA; thus, it becomes feasible to select the biological effects resulting from ISC. We have observed that also FQ forms biadducts between DNA and proteins by a biphotonic mechanism, but, unlike linear furocoumarins, it is incapable of inducing ISC [Bordin et al., 1996]. Therefore, using FQ and the double irradiation, we can selectively induce DPC and analyze their biological consequences. According to our first results, DPC appear to have strong lethal and genotoxic effects in mammalian cells [Marzano et al., 2000]. We observed a marked DNA degradation, which was not detectable just after sensitization, but became evident after 12 hours of post-treatment incubation, probably due to enzymatic processes [Marzano et al., 2000].
Now we report some data obtained comparing FQ to a well-known and very active linear furocoumarin, 4,5’,8-trimethylpsoralen (TMP). Both TMP and FQ can photobind to DNA by UVA irradiation to a high and very similar extent; on the other hand, only TMP shows a strong capacity of inducing ISC [Bordin et al., 1996; Dardalhon et al., 1988]. As a reference compound, we chose 8-methoxypsoralen (8-MOP). The molecular structure of the studied compounds is shown in figure 1.
 |
UVA irradiations were carried out by Philips HPW 125 lamps as described [Bordin et al., 2000]. Compounds were dissolved in dimethylsulfoxide (4.5 mM) and the solutions were kept frozen in the dark. CHO cells were grown and processed as described [Marzano et al., 2000]. DNA damage was studied using alkaline and neutral elution [Kohn et al., 1991] and by isopycnic sedimentation in CsCl gradient. DNA fragmentation was calculated according to Noviello et al. [1994]. The formation of chromosomal aberrations was studied as already reported [Marzano et al., 2000].
RESULTS
Table 1 summarizes the data obtained studying the formation of DPC in CHO cells after a single exposure to UVA light by means of alkaline elution. The data are expressed as dose unit (DU), i.e., normalized as DPC number per million of nucleotides induced by 1 µM concentration and 1 kJ·m-2 of UVA light.
|
Compound |
DPC number (DU·10-3) |
Relative activity |
|
8-MOP |
2.05 |
1 |
|
TMP |
34.2 |
16.7 |
|
FQ |
20.5 |
10 |
 |
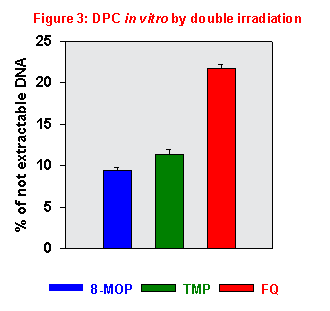 |
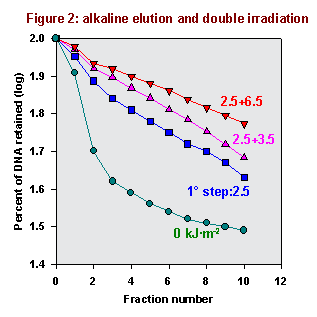
This behavior suggests that TMP, like FQ, induces covalent biadducts linking together DNA and proteins. To investigate this point, we carried out some experiments by isopycnic centrifugation in CsCl gradients.
 |
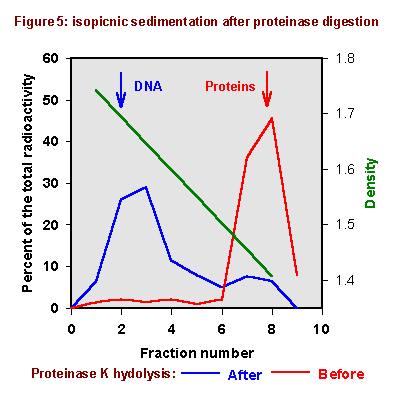 |
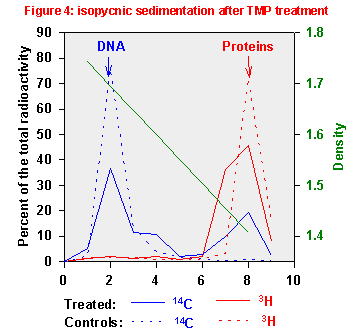
Figure 5 shows the results obtained; they are compared with those achieved
before the proteinase treatment. After protein digestion, almost all 14C
radioactivity bands at DNA density; this means that before proteinase digestion
a part of this macromolecule was covalently bound to proteins.
These results are consistent with the hypothesis TMP also forms bifunctional adducts linking together DNA and proteins, like observed with FQ and 8-MOP [Bordin et al., 1996; 2000].
Then, we compared the main biological responses to DNA damage induced by FQ and TMP. In all these experiments we used the following conditions: drug concentrations: 2 µM; UVA dose 0.12 kJ·m-2 for FQ and TMP; 3.33 kJ·m-2 for 8-MOP
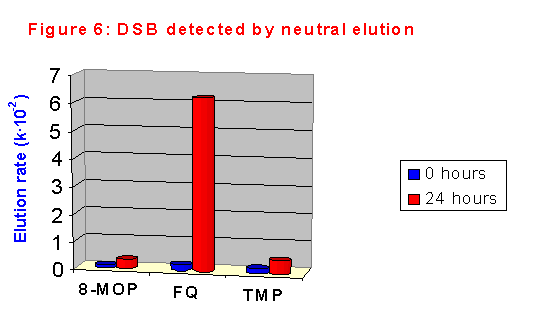
At first we studied the formation of double strand breaks (DSB) in CHO cells using neutral elution. The results are shown in figure 6. As expected, no significant DNA fragmentation could be detected just after sensitization; after 24 hours of post-treatment incubation, FQ induced DSB to a very high extent. Amazing, in spite of its exceptional capacity of damaging DNA, TMP induced a lower amount of DSB, which is very similar to that formed by 8-MOP.
 |
Considering the aberration spectra generated by these drugs, we can observe that FQ and TMP form comparable amounts of chromatide interchanges and isochromatide breaks (blue data). 8-MOP, in spite of its lower activity, induces marked levels of such chromosome alterations.
|
Chromosome aberrations |
|
||||||||||
|
Drug |
Total % |
Dic. |
Rings |
Gaps |
Breaks |
Rings |
Inter-Changes |
Isochr. Breaks |
|||
|
None |
6 |
1 |
- |
- |
2 |
1 |
1 |
1 |
|||
|
FQ |
67 |
1 |
- |
3 |
19 |
5 |
36 |
3 |
|||
|
TMP |
35 |
- |
- |
1 |
1 |
- |
31 |
2 |
|||
|
8-MOP |
24 |
1 |
- |
2 |
5 |
- |
13 |
3 |
|||
However, paying attention to chromatide gaps, breaks and rings, the picture is very different (red data) as TMP seems to be much less effective than FQ. Therefore we could say TMP is less clastogenic than FQ because of its inability to induce such types of chromatide aberrations.
| First Photon | Second Photon | ISC | ||||
| TMP | MA | |||||
| DPC | ||||||
TMP is an exceptionally effective furocoumarin, inducing MA, ISC and DPC, according to the following reaction:
As now it has been revealed TMP forms two kinds of bifunctional adducts, biological responses obtained by various authors with TMP and the double irradiation protocol were incorrectly interpreted as always seen as consequences of ISC alone. Moreover, considering the strong biological effects associated with DPC formation, the conclusions reported by the papers dealing with the biological effects of TMP, [e.g., Laquerbe et al., 1995] must be undoubtedly reconsidered. In this connection, we must point out that DPC seem to be highly genotoxic as observed after FQ sensitization by the double irradiation method [Marzano et al., 2000].
FQ only induces a lot of DPC
| First Photon | Second Photon | |||||
| FQ | MA | DPC | ||||
In fact, differently from TMP, which forms two concurrent kinds of bifunctional adducts, FQ MA can be converted only into DPC. For these features, FQ, used with the method of the double irradiation, can selectively induce these lesions. Therefore, FQ appear to be a helpful derivative for studies on DPC effects.
Do ISC induced by TMP interfere with DPC repair?
At present we have no satisfactory answers to explain TMP behavior; we can only say TMP is less genotoxic than FQ. Considering its high antiproliferative activity and the severe experimental conditions necessary for 8-MOP to achieve comparable antiproliferative effects, we can also suggest that probably TMP might be less dangerous and more suitable for therapeutic uses than 8-MOP itself.
However we can suggest the following hypothesis. According to Rosenstein and Lai [1991], DPC seem to be repaired by topoisomerases. These authors used a frog ICR 2A cell line, very proficient in photoreactivation; the cells were exposed to high doses of light at wavelength greater than 350 nm, which together with DPC, induce reduced extents of dimers. Then, cells were submitted to photoreactivation: thus, pyrimidine dimers were removed, but DPC remained unchanged. During the first two hours of post-incubation, the authors observed an increase in DPC and DNA breaks; in addition, breaks appeared to be associated to DPC so generating typical lesions of topoisomerases. At longer incubation time, both DPC and breaks disappeared. Considering the formation of DSB, we suggest that DPC repair might be accomplished by topoisomerase II activity.
These authors also observed that omitting the photoreactivation step, the not removed dimers hinder DPC repair. This result is consistent with the observations of Pedrini and Ciarrocchi [1983] on the inhibition of topoisomerase I by UV photoproducts.
On the basis of these observations, we suggest that ISC induced by TMP, patching together the two DNA strands, could trap enzymes involved in DPC repair. This could explain the observed differences in induction of DSB and chromosomal aberrations by FQ and TMP.
Ben-Hur,E. and M.M.Elkind, (1973) Psoralen plus near ultraviolet light inactivation of cultured Chinese hamster cells and its relation to DNA cross-links, Mutat. Res., 18, 315-324
Bordin,F., C.Marzano, F.Carlassare, P.Rodighiero, A.Guiotto, S.Caffieri and F.Baccichetti (1996) Photobiological properties of a new tetramethyl furoquinolinone. J. Photochem. Photobiol., B:Biology, 34, 159-168.
Bordin,F., F.Baccichetti, C.Marzano, F.Carlassare, G.Miolo, A.Chilin and A.Guiotto, (2000) DNA damage induced by 4,6,8,9-tetramethyl-2H-furo[2,3-h]quinolin-2-one, a new furocoumarin analog: photochemical mechanisms, Photochem. Photobiol., 71, 254-262.
Dardalhon,M., and D.Averbeck, (1988) Induction and removal of DNA interstrand cross-links in V-79 Chinese hamster cells measured by hydroxylapatite chromatography after treatments with bifunctional furocoumarins, Int. J. Radiat. Biol., 54, 1007-20.
Kohn,K.W., (1991) Principles and practice of DNA filter elution, Pharmac. Ther., 49, 55-77.
Laquerbe,A., E.Moustacchi, D.Papadopoulo, (1995) Genotoxic potential of psoralen cross-links versus monoadducts in normal human lymphoblasts, Mutat. Res., 346, 173-9.
Marzano, C., F.Baccichetti, F.Carlassare, A.Chilin, S.Lora and F.Bordin, (2000) DNA damage induced by 4,6,8,9-tetramethyl-2H-furo[2,3-h]quinolin-2-one, a new furocoumarin analog: biological consequences, Photochem. Photobiol., 71, 263-272.
Noviello,E., M.G.Aluigi, G.Cimoli, E.Rovini, A.Mazzoni, S.Parodi, F.De Sessa, P. Russo, (1994) SCE, chromosomal aberrations and cytotoxycity produced by topoisomerase II-targeted drugs in sensitive (A2780) and resistant (A2780-DX3) human ovarian cancer cells: correlations with the formation of DNA double strand breaks, Mutat. Res., 311, 21-29.
Pedrini,A.M. and G.Ciarrocchi, (1983) Inhibition of Micrococcus luteus DNA topoisomerase I by UV photoproducts, Proc. Natl. Acad. Sci. USA, 80, 1787,
Rosenstein,B.S. and G.Lai, (1991) DNA-protein crosslinking in UV-irradiated human and ICR 2A cell lines, In Photobiology, , Riklis ed., Pergamon Press, NY, pp. 27-34.