Use of uracil thin-layer dosimeter for classification of tanning tubes: hazardous consequences
Z. Kuluncsics, T. Kerékgyártó*, P. Gróf, I. Horkay**, Gy. Rontó
Semmelweis University of Budapest, Faculty of Medicine, Department of Biophysics and Radiation Biology, Hungary;Corresponding author: Zénó Kuluncsics M.D.
Semmelweis University of Budapest, Faculty of Medicine,
Department of Biophysics and Radiation Biology, Hungary
P.O. Box 263, H-1444 Budapest, Hungary
Tel/fax: 36-1-266-6656, e-mail: grof@puskin.sote.hu
Abstract
Although international guidelines and standards limit the application of artificial ultraviolet (UV) sources, sunbed users take higher ultraviolet doses than recommended to reach fast cosmetic effects. Possible consequences depend on the spectral distribution of the sunbed tubes. Here, we describe a method using biological dosimeter to classify different tanning tubes and to predict their possible harmful health effects.
Action spectra of erythema induction and uracil thin-layer dimerization as well as emission spectra of some commonly used sunbed tubes — measured by a spectroradiometer — have been used, to calculate their biological effectiveness. The ratio of the the uracil doses and erythema, HU /HSED, seemed to be appropriate for classification of sunbed tubes. In order to correlate uracil doses and erythema induction times, uracil dosimeters were used in parallel with skin test control on 15 volunteers. Spectral effectiveness of some commercially available tubes with apparently similar emission characteristics was compared using the HU /HSED ratio.
Some of the tubes were found to be more effective in the ultraviolet-B (UVB, 290-320 nm) range, while the others showed similar effectiveness in ultraviolet-A (UVA, 320-400 nm). We determined the recommended exposure time for sunbeds studied. This exposure time ensured that after the first irradiation neither fast cosmetic effects nor erythema induction were observed. However, after the tenth irradiation individually different skin tanning was occurred on the volunteers.
We concluded, that classification of commercial sunbeds as UVB or UVA effective light sources, as well as prediction of their biological effects could also be achieved by a uracil dosimeter. Thus, our results also show that biological dosimeters are suitable to control in every-day use the international health-recommendations and standards specified for sunbeds.
Key words:
Sunbed, UVB, UVA, Ultraviolet radiation, Spectral effectiveness, Uracil thin-layer dosimeter
TEXT
1. Introduction
Exposure to ultraviolet radiation is one of the primary causes of the most common type of cancer, the skin cancer (1-6). More and more epidemiological evidence indicates that artificial tanning lamps also contribute to the risk of skin cancerogenesis (7-8) including malignant melanoma (9-11). Although other hazardous consequences like eye damages, immunosuppression or skin aging have also been reported (12-15), popularity of sunbeds has continued to rise steadily.
Several recommendations and standards were determined for environmental and artificial ultraviolet radiation. UV standard (16) of the National Institute of Occupational Safety and Health (NIOSH) is expressed in Exposure Limit (EL), and it is based on an UV hazard curve (17). It is still the valid standard between 180 and 320 nm. Range of the standardization was extended for UVA range (320-400 nm) (18). For cosmetic use of sunbeds some recommendations were made (19-20), the recommended doses are expressed in Minimal Erythema Dose (MED). Up to now, spectral limitation for the emission of the sunbed tubes have not been constructed; some tubes are commercially available that emit high UVB dose during normal use of the sunbed. Consequently, limitations of the tubes are necessary both in UVB and UVA range. According to the international recommendation, the daily doses of the ultraviolet radiation administered in a sunbed cannot exceed the limit of 1 MED neither for the UVB nor for the UVA ranges (20).
Control of the aforementioned limitation is a difficult task for dosimetry. From point of view of irradiation, spectral distribution in sunbed can be measured with high precision by a spectroradiometer, installing its detector into the irradiation space. However, such measurements were highly expensive for every-day use, and require stabilized measuring conditions. Therefore they are not practical for extensive control of sunbeds. On the other hand, tanning meters - usually based on photochemical reaction - are not expensive and they are easy to use. In fact, their spectral sensitivity is not very similar to that of the biological responses like erythema induction after UV irradiation. This is the reason, why results obtained with tanning meters are not suitable for the prediction of the biological consequences. As broad band physical dosimeter, Robertson-Berger type UV-meters (RB meters) were developed for measuring environmental UV-radiation. In general, these types of pyranometers are available with spectral sensitivity close to the action spectra of the DNA damage or the minimal erythema dose. In all cases, the out-put signal is a voltage level that can be converted by appropriate factors into any biologically effective dose, provided the spectral distribution of the UV source is known with satisfying precision. Due to usual application of RB-meters, they are calibrated for environmental use (21), thereby such devices cannot be recommended for everyday control of artificial UV sources by unskilled personnel. Although, absolute sensitivity of RB-meters were adequate to control sunbeds, its price and maintenance requirements does not allow, at least at present, its daily use.
Requirements for a dosimeter used in control of sunbeds can be thought as: a.) simple use, b.) cumulative dosimetry during individual exposure period, c.) relatively low cost, d.) informative about the relative effective UVB/UVA contribution.
We recommend here a method to determine the biologically effective irradiance in sunbeds based on a biological UV dosimeter developed in our institute: the uracil thin-layer dosimeter (22). One of the most important harmful effects induced by UVB radiation in DNA, is the formation of cyclobutane pyrimidine dimers. Similar dimerization photoreactions occur in polycrystalline uracil thin-layer (23). Uracil dosimeter measures the biologically effective UV radiation with spectral sensitivity very close to the DNA absorption spectra (24). The method proposed in this work, however, has limited validity for sunbeds emitting only UVA, because of the lower sensitivity of the uracil dosimeter at UVA wavelength. For this case other biological dosimeters, like T7 bacteriophage or Bacillus subtilis spore, could be applied (25-27).
2. Methods
2.1 Tubes investigated
Ten commercially available sunbed tubes have been used for investigation. The tubes were produced by different companies. These are as follows: Bellarium S., Beauty Sun, Euro Sun and Hellarium tubes from Wolff System; TL Professional from Philips; Bermuda Gold, Super Bronz and Super Gold from Lighttech; Brillant Sun Plus and Goldarium S. from Cosmedico. Tubes were encoded randomly as T360-T369.
2.2 UV dosimetry
For preparation of polycrystalline uracil thin layer, Edwards Auto306 vacuum coating system has been used. Biologically effective UV dose determined by uracil dosimeter can be expressed in HU units; 1 SED (standardized minimal erythema dose) corresponds to about 2.5*10-3 HU for a spectral distribution equivalent to that of the environmental radiation which can be measured at fine summer days at temperate zone. Spectral sensitivity of the uracil dosimeter, its preparation, checking and evaluation has been published previously (28).
When separated tube was measured, the dosimeters were irradiated at parallel position, 5-cm distance from the geometrical middle of the tube, during 72 hours.
The relative emission spectra between 250 and 400 nm of three different sunbed tubes were determined in our laboratory by a Jobin-Yvon THR 1000 single monochromator (bandwidth 0.2 nm; step increment 0.1 nm) and equipped with a Hamamatchu R928 photomultiplier. To have the best possible dynamic resolution for the whole 250-400 nm interval, two different amplification have been used, a higher one for the 250-310 nm region and a lower one between 310-400 nm. Typical emission spectra of three sunbed tubes are shown in Figure 1. The three spectra are very similar, although the spectrum of T367 lamp contains more short-wavelength components than T362 and T364. The UVB% of the spectra are given in the first column of Table 2.
2.3 Classification of the sunbed tubes
Biologically effective UV dose-rate was determined by spectrophotometric measurement using uracil dosimeters (28). Ratios of dose-rates (HU/hour)/(HSED/hour) were then calculated for each of the sunbed tested. As it is given later, the higher the calculated ratio is, the greater is the biologically effective UVB contribution.
In order to establish a relation between calculated HSED and measured HU values, relative spectral irradiance (E(l )) of the investigated lamp as well as the corresponding HU dose have been measured. Knowing the standardized erythema spectral sensitivity (SSED(l )) as well as that of uracil dimerization (SU(l )), biologically effective UV dose expressed in HSED was calculated according to equation (1):
![]() , (1)
, (1)
where w(
l)i is a weighting factor; and r(l)i denotes ratios of spectral sensitivities (SSED(l)i/SU(l)i) as the function of the wavelength. The weighting factor in equation (1) can be defined as: 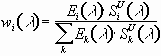 (2).
(2).
Combining equations 1. and 2. one can derive the well known equation to transform a certain biological UV dose into other one (29). Equations 1. and 2. express the fact that transformation between any two units depends on the spectral distribution of the UV source and the sensitivity ratios as well.
2.4 Determination of recommended irradiation time in sunbeds
Because of its small size, uracil dosimeter is suitable to measure the UV dose in sunbed by placing it at the user’s position. Sunbed A was equipped with 40 tubes T362, sunbed B is equipped with 36 tubes T364 and sunbed C with 24 tubes T367. Dose rate values for HU/24h have been directly measured by uracil dosimeters. Three dosimeters were placed inside the sunbed on the surface of a phantom: on the face, on the back and on the chest. The highest among the three measured dose was used to determine the administered dose rate. At each sunbed HSED/h values were calculated according to (1).
2.5 Skin tests
Irradiation time for development of the erythema was determined by skin tests on volunteers with skin type 1 and 2, with the aim to check the dose in sunbeds. According to the recommendations (19-20), exposure can not exceed 1 MED. MED rates for the skin tests were pre-calculated on the basis of dose-rates previously measured by uracil dosimeters. On the back of volunteers 5 round skin surfaces with a diameter of 4 cm were irradiated with increasing doses. The middle spot received the estimated 1 MED; the other doses differed by -40%, -20%, +20 and +40% from the middle spot. The skin-response was investigated 12 hours after the irradiation. Developed erythema was denoted by plus sign. If the previous calculation was correct only the last two or three spots became positive. The irradiation time of the last negative spot was chosen as the recommended sunbathing time.
The recommended exposure time of sunbed does not cause fast cosmetic effect. To verify if repeated utilization can produce tanning without any side effect, volunteers were irradiated during the recommended time, twice a week as long as 5 weeks.
3. Results AND discussion
3.1 Laboratory measurements on sunbed tubes
3.1.1. Uracil doses, which can reflect dimerization of pyrimidine bases, were determined for 10 different, commercially available sunbed tubes. The results are summarized in Table 1. These data were obtained with new tubes: in the first series they were used for irradiation during 72 hours. In repeated series, dose was measured with the same tubes during an additional 72 hours irradiation. Although in all cases but two a decrease in HU dose was detected, a statistical analysis of these data showed that there is no significant difference between the first and the second 72h doses. Thus, aging of the tubes and the accompanying shift in the spectral irradiance can be expected only after a longer utilization period. On the other hand, tubes T365 and T362 are two-three times more effective in dimerization of uracil than tubes T368, T366 and T364, which is out of the experimental errors, corresponding to about 10-15% for the given dose-interval (29). Figure 2 gives a comparison among sensitivities of the RB-meter used in our experiments, the uracil dosimeter as well as those for standardized minimal erythema dose and phage T7. An inspection of these spectra proves that uracil dosimeter possesses a higher relative sensitivity at shorter wavelengths than the RB-meter used. Thus, uracil dosimeter indicates with a high sensitivity the short-wavelength components in the emission spectra of the tubes. Due to spectral sensitivity of the uracil dosimeter, these differences call attention to the importance of the contribution from short-wavelength components in the spectra of these tubes.
3.1.2. Without the appropriate biological spectral sensitivity, even a spectroradiometric measurement alone cannot estimate biological effectiveness of a given UV source, but it characterize its « physical nature ». However, according to the recommendations for characterizing sunbeds (19-20), qualification of a tube can be based on the SED effectivity due to UVB versus UVA proportion of the UV radiation. Based on the results presented in Table 1, three different sunbed tubes were selected for further analysis: tube T362 with high-, while tubes T364 and T367 with lower uracil effectiveness. Areas, which correspond to UVB or UVA regions under the effectiveness spectra (not shown here), are proportional to the biological effects (e.g. dimerization, eryrthema induction) caused by the different regions of UV radiation. Wavelength dependence of relative biological effectiveness for these tubes was calculated taking SED and uracil spectral sensitivities. As an example, Fig. 3 shows the corresponding relative effectiveness for T364 (similar dependence has been found for T362 and T367): depending on the given photobiological reaction, different intervals of the whole UV spectrum contribute in different extent to the total biological effectiveness. Comparing the UVB and UVA contribution on Fig. 3, much higher proportion of dimerization occurs in UVB (~99%) than in UVA (~1%) as it can be expected also on the basis of CPD formation. On the contrary, UVB (~50%) and UVA (~50%) parts show similar contribution in the biological effectiveness of skin reddening. The ratios of biological effectiveness due to UVB and UVA for erythema induction and uracil dimerization are shown in Table 2. Thus ratios for skin reddening remain in the same order of magnitude (1.83, 1.01 and 1.78 respectively for T362, T364 and T367); for uracil dimerization this ratio varies between 2 and 3 orders of magnitude (772, 129 and 145 respectively).
Formation of different pyrimidine photoadducts is more important in UVB region, while photo-oxidative damages, erythemal effects result in to relatively higher extent due to UVA radiation (30). Because both UV regions provoke harmful health effects, neither UVB nor UVA contribution should be underestimated by a methodology aimed to characterize sunbed tube’s efficiency. To minimize health risk either with shorter-, or longer manifestation time, both UVB and UVA load should be decreased to an appropriate, controlled level. To qualify sunbed tubes, we propose to apply a combined method using two well-characterized responses with the corresponding action spectra. Classification of tanning tubes can be based on limitation separately on UVB and UVA effectiveness or on the ratio of effective UVB to effective UVA. In the latter case the effective UVB and UVA doses should be measured with a dosimeter having similar spectral sensitivity as the DNA and the minimal erythemal effect respectively. For example, use of two RB meters, one possessing DNA sensitivity and a second one with MED sensitivity could be a solution to solve this problem.
A comparison of the shape of the spectral sensitivities (Fig. 2) for uracil dimerization with standardized minimal erythema dose shows, that for UVB region the uracil dosimeter is relatively more sensitive and closer to DNA action spectra, while erythemal effect is relatively more important in UVA region. The ratios HU/HSED were calculated for each tube. These values are indicated in the last column of Table 2, and they can be considered as a characteristic of the tubes. Biologically effective uracil dose, HU, was determined by direct measurements, while the standardized effective erythemal dose, HSED, was calculated on the basis of spectroradiometric measurements using the erythemal action spectrum. Definition of a ratio HU/HSED can be a tool to classify a tube. A higher value of the ratio HU/HSED would indicate a greater contribution due to UVB radiation. Thus, according to Table 2, the tube T362 possesses a higher biological effectiveness from UVB than the others, while T364 has more pronounced effectiveness due to UVA region. It should be noted however, that due to the characteristic of the appropriate spectral sensitivities, uracil dosimeter would underestimate erythemal effect due to UVA, while an RB-meter with MED spectral sensitivity can underestimate the dimerization effect due to UVB. HU/HSED has been determined for environmental radiation, measured from May to August 1995 in middle Europe (47.5° latitude), it gives a value of 2.5 for the HU/HSED*103 ratio (31-32).
3.2. Medical-technological analyses of sunbeds based on current recommendation
3.2.1. Table 3, presents the measured HU/24h dose rates (0.50, 0.20, 0.40 respectively) that corresponds to the photodimerisation capability of three different types of sunbeds (A,B and C) containing tubes T362, T364 and T367 respectively. Exposure time for sunbathing was determined based on the currently valid WHO/UNEP/IRPA (19-20) recommendation, which defines a threshold of 1 MED. The measured uracil doses were used to calculate the appropriate maximal exposure times for different sunbeds. Conversion of uracil doses into MED values and calculation of the allowed exposure time can be achieved by equation 1. Calculation based on equation 1 and the measured HU dose rates resulted in the recommended times in sunbeds A, B and C to be 16, 13 and 8 minutes, respectively. As it can be seen from the given times, despite of classification of a tube the precise exposure threshold time in a sunbed is further influenced by the difference in geometrical condition, number and power of tubes. According to these, although the measured dose rates and international recommendations based on erythema production allowed the longest exposure time in sunbed A, its tubes was found to be the most effective among the tubes in formation of cyclobutane pyrimidine dimers. This finding can be also a hint that beside of minimizing erythemal effects of sunbeds, other health risks should have been taken into account as well, in order to decrease hazardous consequences of sunbeds.
3.2.2. The required individual UV exposures at hospitalised persons are usually determined by a series of different irradiation times or controlled intensities and subsequent observation of the cutaneous reaction. These methods take into account the individual differences even with similar skin types, thus it allows a precise dosage of UV radiation. Under the conditions can be found in public use of sunbeds, we propose a more simple solution as it is outlined in the followings. The UV dose-rates, measured by uracil dosimeter can be converted into SED values taking into account the spectral irradiance. These SED values can be used to determine the recommended exposure time. To prove the validity of our proposal, dermatological investigations were performed to determine the recommended exposure time and control the validity of the calculated values. In order to prevent overexposure, development of the erythema on volunteers with skin types 1 and 2 has been assessed 12 hours after the exposure. Five volunteers were exposed in each sunbed for five different irradiation times: 1.) 1 SED (i.e. to induce erythema), 2.) and 3.) 1±0.2 SED, 4.) and 5.) 1±0.4 SED. The results are summarized in Table 4. None of the 15 volunteers had erythema after exposure with 1 SED or 20%, 40% less. 11 volunteers of the 15 received skin reddening after an exposure 20% more than calculated 1 SED. 1 of the 15 volunteers did not developed erythema at all. These results indicate that none of the volunteers have got erythema after treating them with the recommended exposure time. Although, all but one volunteers had erythema after the 1.4 SED, the strength of the biological response was greatly different indicating that individual differences have of great importance. To control the cosmetic effectiveness of the recommended irradiation times and to observe any side effects, repeated whole body irradiation was performed twice a week on volunteers. After the tenth treatment, skin tanning was observed in every case. Its strength individually varied. Side effect was not observed.
4. Conclusion
The use of sunbeds for cosmetic purposes is generally not recommended (19-20). According to the recommendation of International Radiation Protection Association / International Non-Ionizing Radiation Committee (IRPA/INIRC) (1989) no visible changes should be detected on the skin or on the eyes after exposure less than the daily limit. The aim of sunbed users is, however, to become tanned fast, which from its side is also a visible change of the skin, so higher dose than the daily limit is usually administered. We showed here the possibility to become tanned keeping the recommended irradiation time at the qualified UV sources. However, also in this case not all of the hazardous effect of this irradiation can be excluded. It is well established that artificial tanning lamps induce harmful, stochastic consequences: like skin cancer or cataract. Furthermore, studies suggest that repeated exposure with about 70% of the MED induce immunosuppression (12).
The development of the erythema is the same after UVA and after UVB treatment, but the mechanism of becoming tanned are different (33). The sunbed tube causes immediate, direct suntan, while the sun induces indirect suntan after 2-3 days. Furthermore, it has been shown that protection efficiency of the tan induced by UVA lamps is minor compared to tan produced by UVB lamps or natural sunlight (34). Consequently, sunbeds are not recommended to prepare the skin for higher solar exposure before a summer holiday.
Emission spectra of the tubes older than the recommended 500-750 hours could be shifted towards shorter wavelengths, while their cosmetic effectiveness can decrease by aging through a decrease in their emitted power. In this situation the sunbed user increases the exposure time to get the same cosmetic responses, thereby also the dose due to the UVB radiation increase. For long-term control we suggest to place uracil dosimeter continuously inside the sunbed. Under optimal conditions, less than 60% decrease of the optical density of the uracil has to be observed after 500 hours use of the lamps (35). Furthermore, it were advisable to control the replacement of the tubes by new ones after the industrially prescribed 500-750 hours usage should be worth to control.
The emission properties of various types of tanning tubes are different. Classification of the sunbed tubes by controlling their spectral properties is required; in order to follow-up the UV load on population-level due to hazardous artificial light sources. Based on our results presented here, the HU/HSED ratio was found as a good characteristic of tanning tubes. The higher is this ratio the greater part of the biological effectiveness comes from the UVB range. It is well established that after UVB irradiation the health consequences are likely due to direct excitation of DNA, which results in mainly bipyrimidine products, like the cyclobutane pyrimidine dimers, /6-4/ photoproducts and Dewar isomers (36-38). On the contrary, if the HU/HSED ratio is low the hazardous effects of the sunbed are likely due to UVA radiation. It is believed that UVA is not absorbed directly by DNA but may produce photoreactions indirectly through the generation of reactive oxygen species and the photosensitizer agents (39-46). This type of mutagenic DNA damage induction was recently found to be more important after exposure to artificial tanning lamps than to natural sunlight (47). In coincidence with these potentially carcinogenic mutations, a recent case report demonstrated the induction of non-melanoma skin cancer associated with use of a tanning bed (48).
Finally, it has been clearly demonstrated here that there is no direct relation between the potential DNA damage inducing effect of the sunbed and the internationally recommended (WHO/UNEP/IRPA) exposure time. Recommendations based on erythema induction is limited to control and prevent health risk with dissimilar action spectra, like skin cancerogenesis, developing cataract or immunosuppression. Therefore, establishment of a new international recommendation for limitation of sunbeds based on action spectra of other hazardous health consequences should be welcomed.
ACKNOWLEDGMENT
The authors are indebted to Mr. S. Gáspár for helpful discussions. Also, we gratefully acknowledge Mr. L. Herényi for help in performing the measurements of the emission spectra of the tubes and Mr. Z. Filep for supplying the tubes. This work was supported by EU, "BIODOS", ENV4-CT95-0044, EU, "UVB CELLS", ENV4-CT95-0174, a grant of Hungarian Ministry of Education, FKFP 1190/1997, a grant of Hungarian Ministry of Health and Welfare, ETT T-08 328/93 and a student grant from the KGy Solar Kft.
References
A Recommended Standard for Occupational Exposure to Ultraviolet Radiation. U.S. Department of Health, Education, and Welfare: Washington, D.C., 1972 (U.S. Government Printing Office No. 017-033-00012).
Tables
Table 1. Uracil doses measured at different sunbed tubes in a distance of 5 cm. Doses are given in uracil units (HU)
|
number |
HU (first 72 hours) |
HU (second 72 hours) |
|
1.24 |
1.31 |
|
|
T361 |
1.19 |
1.16 |
|
T362 |
1.46 |
1.38 |
|
T363 |
1.21 |
1.13 |
|
T364 |
0.71 |
0.62 |
|
T365 |
1.67 |
1.69 |
|
T366 |
0.64 |
0.65 |
|
T367 |
0.87 |
0.78 |
|
T368 |
0.52 |
0.50 |
|
T369 |
1.38 |
1.30 |
Table 2. UVB proportion of the emission spectra, ratio of UVB/UVA effectiveness for erythema induction (MED) and dimerization (U) of different tubes, and HMED/HU values for sunbed tubes classification
|
type |
UVB% |
HMEDUVB/ HMEDUVA |
HUUVB/ HUUVA |
HU/HMED*103 |
|
T362 |
1.85 |
1.83 |
772 |
5.49 |
|
T364 |
1.73 |
1.01 |
129 |
1.74 |
|
T367 |
3.37 |
1.78 |
145 |
2.31 |
Table 3. Calculation of recommended exposure time for sunbed A equipped with 40 tubes T362, sunbed B equipped with 36 tubes T364, and sunbed C equipped with 24 tubes T367 based on HU/HMED tube classification and dose rate measured by uracil (HU/24h)
|
sunbed |
HU/HMED*103 |
HU /24h |
HMED /h |
time for 1MED |
|
A |
5.49 |
0.50± 0.02 |
3.79 |
16 min. |
|
B |
1.74 |
0.20± 0.01 |
4.78 |
13 min. |
|
C |
2.31 |
0.40± 0.02 |
7.22 |
8 min. |
Table 4. Development of erythema on skin type 1 and 2 volunteers after exposure by 0.6, 0.8, 1, 1.2 and 1.4 SED
|
sunbed |
0.6 SED |
0.8 SED |
1 SED |
1.2 SED |
1.4 SED |
|
A |
0/5 |
0/5 |
0/5 |
4/5 |
5/5 |
|
B |
0/5 |
0/5 |
0/5 |
4/5 |
4/5 |
|
C |
0/5 |
0/5 |
0/5 |
3/5 |
5/5 |
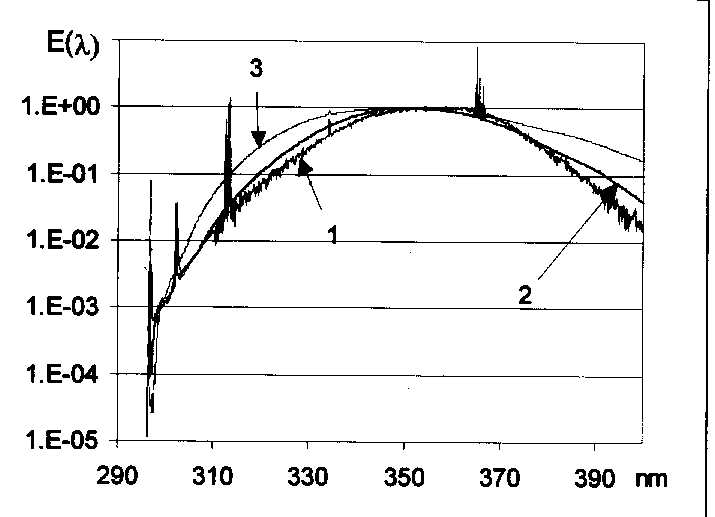
Fig. 1, Emission spectra of three sunbed tubes. The three spectra are very similar, although the spectrum of T367 lamp (black line) contains more short-wavelength components than T362 and T364 (gray and light gray lines respectively).
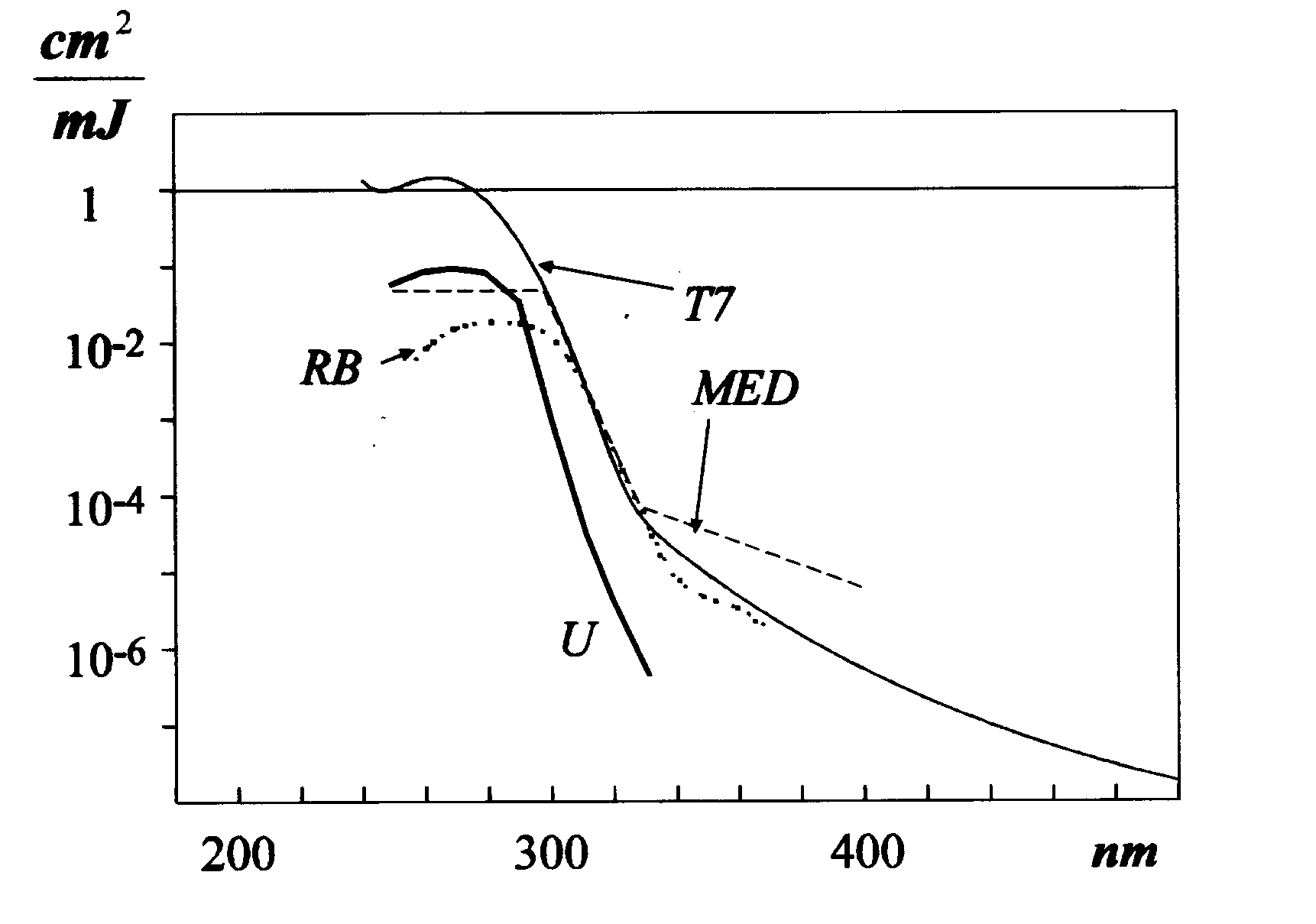
Fig. 2, Absolute spectral sensitivities, expressed in cm2/mJ of the uracil dosimeter (U), the RB-meter (RB), the erythema (MED) and T7 bacteriophage (T7).
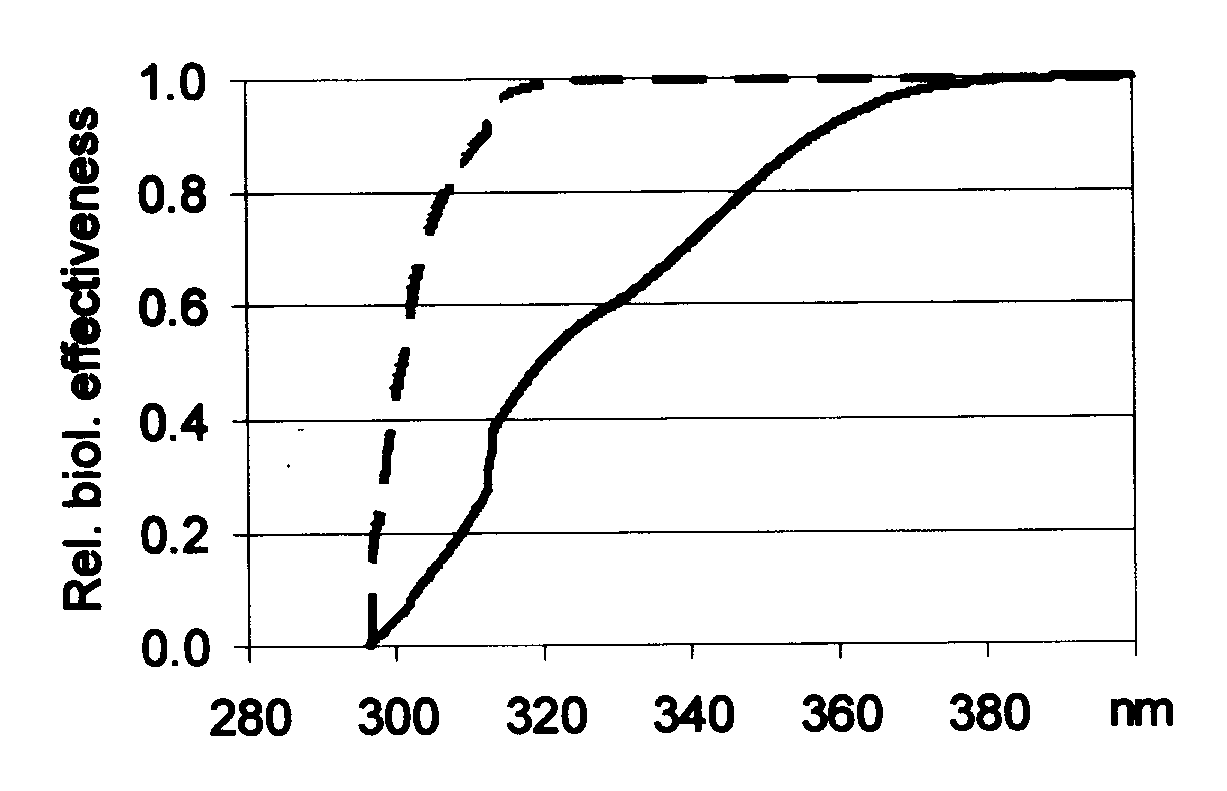
Fig. 3, Wavelength correspondence of the relative biological effectiveness of tube T364 on uracil dimerization (scattered line) and erythema induction (full line). Results presented here on cumulative curves.