| Site home page | Conference home page | Discussion |
COMPARISON OF PHOTOHEMOLYTIC EFFICIENCIES OF DEUTEROPORPHYRIN-IX DERIVATIVES
Galina V. Mansurovaa, Oksana G. Pogrebnayaa, Gelii V. Ponomarevb, Andrei V. Reshetnickovb, Alexander Ya. Potapenkoa*, Lina N. Bezdetnayac and François Guilleminc.
a Department of Medical and Biological Physics, Russian State Medical University, Moscow, 117869 Russia
b V.N.Orekhovich Institute of Biomedical Chemistry of Russian Academy of Medical Sciences, Moscow, Russia
c Unite de recherche en Therapie Photodynamique, Centre Alexis Vautrin, Vandoeuvre-les-Nancy, 54511 France
*Corresponding author. e-mail: potap@hotmail.com
The abilities to photosensitize hemolysis of human erythrocytes of 4-(1-methyl-3-hydroxybutyl)-deuteroporphyrin-IX (I) and 2,4-di(1-methyl-3-hydroxybutyl)-deuteroporphyrin -IX (II) have been compared. The photohemolytic efficiency of dye I was shown to be about 60 times higher than that of dye II. It was found that a part of each dye tightly binds the erythrocyte membranes and cannot be removed by washing. The method is proposed for the estimation of the share of the dye tightly bound to the membrane (b) with screening effect produced by free dye and photohemolytic efficiency of the bound dye being taken into consideration. The values of b for dyes I and II are shown to be 86% and 61%, they are shown to correlate with the values of their distribution coefficients for the system 1-octanol:phosphate buffer, pH 7.4, making 20.7 and 17.0, respectively.
INTRODUCTION
Porphyrin derivatives combined with the exposure to visible light are used as photosensitizers (PS) in the photodynamic therapy of tumors [1]. One of the key mechanisms in the death of tumor cells in PDT is the damage to cell membranes [2]. To evaluate the membranotoxic properties of PS, photohemolysis of erythrocytes is frequently used as a well-studied and reproducible model [3]. Generally, the hemolysis develops by the colloid-osmotic mechanism, it may be described by the following equation [4]:
V=V0 +aDb (1)
Here V and Vo are the rates of the photosensitized and dark hemolysis, respectively, equal to 1/t50, t50 being the time required to achieve the lysis of 50% cells. D is fluence. The coefficient a before D characterizing the hemolytic efficiency of a photosensitizer depends on PS concentration, molar absorption coefficient for the present wavelength value, the quantum yield of the photochemical reaction, and on PS binding to the membrane in the vicinity of the critical target. Power b indicates the number of light quanta required by one act of membrane damage [4].
When PS is added to erythrocyte suspension, a part of it binds to the membrane, the unbound part is solved in the suspension medium. In photohemolysis, the PS bound to the membrane is responsible for membrane damage, while the unbound PS is not active [5].
It is generally accepted to determine the octanol/water distribution coefficient characterizing the reversible binding at equilibrium, in order to estimate PS ability to bind to the membrane. However, in the case of biological membranes, this binding is not always reversible. If PS is tightly bound to the membrane, it cannot be completely washed from the cells by means of centrifuging or transferring in a new suspension medium. This is frequently used in the studies of the photodynamic damage of cells in vitro. The cells are washed to remove the excess of PS, in order to decrease the effect of the optical screening by the inactive PS forms [6].
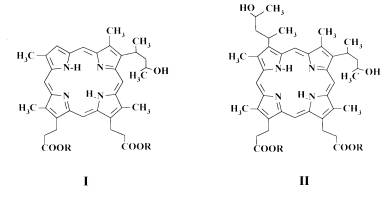
In the present paper the abilities to bind tightly to erythrocytes of the water soluble PS, 4-(1-methyl-3-hydroxybutyl)-deuteroporphyrin-IX (I) and 2,4-di(1-methyl-3-hydroxybutyl)-deuteroporphyrin-IX (II), were estimated (Fig. 1).
 |
Fig. 1. Structural formula of 4-(1-methyl-3-hydroxybutyl)-deuteroporphyrin-IX (I) and 2,4-di(1-methyl-3-hydroxybutyl)-deuteroporphyrin-IX (II). R = N-methyl-D-glucamine.
MATERIALS AND METHODS
The investigated PS 4-(1-methyl-3-hydroxybutyl)-deuteroporphyrin-IX and 2,4-di(1-methyl-3-hydroxybutyl)-deuteroporphyrin-IX were synthesized as described elsewhere [7].
Human erythrocytes were obtained from fresh blood. The cells were twice washed by centrifuging at 400 g. After centrifugation, the cells were placed in the saline phosphate buffer solution PBS (“ICN Biomedical inc.”) with pH 7.4; erythrocyte concentration was adjusted to 107 cells/ml.
The samples were irradiated with a Narva 125-2 lamp (Germany), emission maximum being 365 nm. Simultaneously 8 samples could be exposed to irradiation and stirred with magnetic stirrers. Light intensity at 365 nm measured with a calibrated photocell “Waldman UV-meter” (Germany) was 26 W/m2.
Hemolysis was registered by the change in the suspension turbidity in the red light (670 nm) using a KFK-2MP photoelectric colorimeter as described in [8].
The results treated with variation statistical methods are presented as the mean value ± standard error of the mean (SEM).
RESULTS AND DISCUSSION
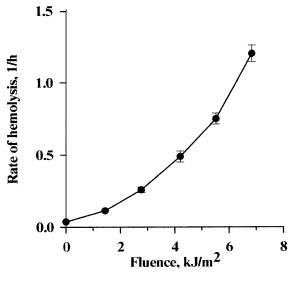
In Figure 2 the fluence dependence of the rate of photohemolysis sensitized by dye I is presented. Such dependence was also obtained for hemolysis sensitized by dye II (the curve is not presented). The power of the fluence value (b) for dyes I and II was 1.90+0.05 and 1.85+0.03, respectively. These values are close to 2. It is assumed that if b is equal to 2, then, a cationic channel is formed due to the independent damage to 2 subunits of the band 3 membrane protein [5].
The coefficients of hemolytic efficiency of dyes I and II were a1=(36,2±3,2).10-3 м4/(h J2) и aII=(0,6±0,04).10-3 м4/(h J2).The hemolytic efficiency of dye I was 60 times higher than that of dye II.
 |
It is known that PS bound to biomembranes cannot be washed off completely by changing the suspension medium [6], i.e., a certain portion of PS remains tightly bound to the membranes. To estimate the share of PS bound to the membrane, the cells should be washed in a fresh suspension medium, and the photosensitizing effects of PS in the washed samples and the samples, which were not washed, should be compared. However, along with the removal of the membrane-bound active dye, the inactive free PS, conditioning the optical screening effect, is removed as well. Thus, the washing procedure exerts two opposite effects: a decrease in the hemolysis rate, achieved through the removal of a part of the active PS, and an increase in the hemolysis rate by means of the decreased screening.
The screening effect can be calculated and taken into consideration. Or under experimental conditions, it can be diminished by the lowered absorbance of the suspension. To do this, it was reasonable to decrease the thickness of the irradiated solution.
Both approaches were used in the estimation of the share of PS tightly bound to the membranes.
The tightness of dye binding to erythrocytes was determined by the following scheme: erythrocyte suspension with added PS was incubated in darkness, then, divided into equal parts by volume and centrifuged. One sample was subsequently resuspended in the same supernatant, and the supernatant of the other sample was replaced by phosphate buffer solution without a dye. Then, both samples were irradiated, and hemolysis was registered.
 |
 |
||
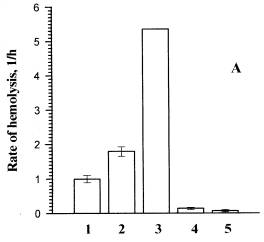
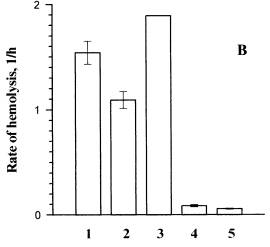
Fig. 3. The influence of erythrocytes washing from dye I on the hemolysis rate.
A ) the thickness of the suspension sample is 1cm, B) the thickness of the suspension sample is 0.1cm. The incident fluence at l=365 nm was 6.24 kJ/m2. The temperature of cell suspension during the pre- and post-irradiation incubation was 37oC. All the samples were centrifuged. 1 and 2 are the rates of photohemolysis in the washed sample and in the sample that was not washed, respectively.4 and 5 are the rates of dark hemolysis in the washed sample and in the sample that was not washed, respectively. 3 is the rate of photohemolysis in the sample that was not washed corrected taking into consideration the screening effect.
In our experiments, to lower the screening effect we shortened the optical pathway of light, by using thinner cuvettes for sample irradiation (Fig. 3b). In the suspension washed from the dye (column 2) the rate of hemolysis was by 30% lower than that in the suspension that had not been washed (column 1). Thus, in this experiment the screening effect was considerably less pronounced than in a thick cuvette (Fig. 3a). Although, a part of PS remained tightly bound to the membranes, and the other part was not bound and was removed by washing, which conditioned a decrease in the hemolysis rate. We worked out the method for calculation of the share of PS bound to the membranes at the critical target. This method takes into consideration both washing from the free PS (increase of the photohemolysis rate due to decrease in screening) and from a part of the bound PS (decrease of the photohemolysis rate).
We suggest the formula for calculation of b - the share of the PS tightly bound to the membranes (See Appendix):
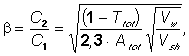 (2)
(2)
where C1 is the amount of PS initially bound to the membrane; C2 is the amount of PS tightly bound to the membrane, i.e., remaining bound after the washing; Ttot and Atot are the transmission and absorbance of PS in the suspension before the washing; Vw and Vsh are the rates of hemolysis in the washed sample and in the sample which was not washed, respectively.
It can be shown that 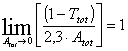 ,
i.e., in the diluted or extremely thin solution samples, the screening effect
is absent. In the optically dense solution samples the ratio
,
i.e., in the diluted or extremely thin solution samples, the screening effect
is absent. In the optically dense solution samples the ratio ![]() may
be used as the correction factor for screening.
may
be used as the correction factor for screening.
As it is shown in Fig. 3, considering the correction factor for screening increased the difference between the rates of hemolysis in the washed samples (columns 2) and the samples that were not washed (columns 3). The calculations by the formula (2) showed the share of the tightly bound dye I to be 86%.
The same measurements (data are not presented) and calculations were carried out for dye II. In our experiments the rates of photohemolysis in the 1 cm and 0.1 cm thick samples decreased by 20% and 60%, respectively, after washing. The screening effect is observed to be more pronounced in the thicker samples. Though in both samples the decrease in the hemolysis rate caused by washing off a part of the bound PS was predominant. The share of dye II tightly bound to the membrane, as determined by the formula (2) made 61%.
The distribution coeffecients for dyes I and II in the octanol/water system were 20.7 and 17.0, respectively. It is clear that tightness of the binding (b) for the dyes correlates with the value of the distribution coefficient (K). However, the difference between hemolytic efficiencies (a) of the two dyes is much greater than the differences between their b and K. Apparently, the hemolytic efficiency is mainly determined not by the dye ability to bind the membrane but by the location of the dye in the membrane in the vicinity of the critical target, presumably, near the band 3 membrane protein.
APPENDIX
Because V is much higher than Vo, Eqn. (1) takes the following form:
![]() (3)
(3)
The fluence absorbed by the membrane-bound PS may be calculated in the following way:
![]() (4)
(4)
where Iabs is light intensity absorbed by the active PS, t is time of irradiation.
Similarly to calculation of screening effect used in the fluorescence analysis [9], the expression for intensity of the light absorbed by the membrane-bound dye in the presence of the screening mixture Iabs, sh will be the following:
![]() (5)
(5)
where Io is the intensity of the incident light; A is absorbance of membrane-bound PS.
After the removal of the screening PS from the solution, the expression for Iabs takes the form:
![]() (6)
(6)
To estimate the share of PS bound to the membrane, taking into consideration that the rate of hemolysis is proportional to squared PS concentration [4], we can derive:
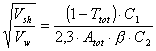 (7)
(7)
Finally we have the formula (2) for obtaining the coefficient b.
Acknowledgements. This study was supported by the Russian Foundation for Basic Research (grant No. 98-04-49054).
The authors are grateful to Dr. E. P. Lysenko for construction of the set-up for irradiation of samples, and to Dr. A. M. Tikhomirov for discussion of the data.
REFERENCES
[1] Brault D., Molecular aspects in tumor photochemotherapy, Laser. Med. Sci. 3 (1988) 41-45.
[2] Dougherty T.J., Gomer C.J., Henderson B.W., Jori G., Kessel D., Korbelik M., Moan J., Peng Q., Photodynamic Therapy, J. Nat. Cancer Inst. 90 (1998) 889-905.
[3] Lagerberg J.W.M., Williams M., Moor A.C.E., Brand A., Van der Zee J., Dubelman T.M.A.R., Van Steveninck J., The influence of merocyanine 540 and protoporphyrin on physicochemical properties of the erythrocyte membrane, Biochim. Biophys. Acta 1278 (1996) 247-253.
[4] Pooler J.P., The kinetics of colloid osmotic hemolysis. Photohemolisis, Biochim. Biophys. Acta 812 (1985) 199-205.
[5] Pooler J.P., and Girotti A.W., Photohemolysis of human erythrocytes labeled in band 3 with eosin-isothiocyanate, Photochem. Photobiol. 44 (1986) 495-499.
[6] Melnikova V., Bezdetnaya L., Belichenko I., Merlin J.-L., Potapenko A., Guillemin F., Meta-tetra(hydroxyphenyl)chlorin-sensitized photodynamic damage of cultured tumor and normal cells in the presence of high concentrations of alpha-tocopherol, Cancer Lett. 139 (1999) 89-95.
[7] Reshetnickov A.V., Zhigal'tsev I.V., Kolomeichuk S.N., Kaplun A.P., Shvets V.I., Zhukova O.S., Karmenyan A.V., Ivanov A.V. and Ponomarev G.V. The preparation and some properties of a liposome formulation of 2,4-di(1-methyl-3-hydroxybutyl)deuteroporphyrin IX. Russ. J. of Bioorg. Chem. 10 (1999) 782-790.
[8] Bezdetnaya L.N., Potapenko A.Ya., Perkhova O.Yu., Nagiev A.I., Sukhorukov V.L., Vladimirov Yu.A., Photosensitized lesion of erythrocyte membrane induced by psoralen: two mechanisms, Biologicheskie membrani (Russ.) 4 (1987) 270-279.
[9] Vladimirov Yu.A., Potapenko A.Ya., Physico-chemical grounds for photobiological processes (Russ.), Vysshaya shkola, Moscow (1989)