|
 |
| Site home page | Conference home page | Discussion |
THERMAL DIFFUSION COEFFICIENTS OF SOLID POLYMERIC LASER DYE SOLUTIONS: A TIME-RESOLVED THERMAL LENS STUDY
Manuel Pons1, Santi Nonell1, Inmaculada Garcia-Moreno2, Angel Costela2 and Roberto Sastre3.
1
Molecular Engineering Group, Institut Químic de Sarrià, Universitat Ramon Llull;2
Instituto de Química Física Rocasolano, CSIC, Serrano, 119, 28006 Madrid (Spain)3
Instituto de Ciencia y Tecnología de los Polímeros, CSIC, Juan de la Cierva, 3, 28006 Madrid (Spain).
ABSTRACT
We have applied the time-resolved thermal lens technique to determine the local thermal diffusion coefficients of solid polymer-glass solutions of rhodamine 6G, which are used as an alternative to conventional liquid-solution dye lasers.
The matrices assayed were copolymers of methyl methacrylate (MMA) and 2-hydroxyethyl methacrylate (HEMA) (1:1 and 7:3 v/v) in which the dye was either dissolved or incorporated covalently bound to methacrylate through a benzylic ester linkage. Previous studies have shown that the polymer-matrix composition and the covalent binding of the dye to the polymeric chain have a substantial effect on the lifetime of the material (measured as the number of pulses that produce a 80% drop of the laser output).
Our results indicate that the local thermal diffusion coefficients:
We conclude that the reported differences in the long-term thermal and photo-stability of the laser materials are not related to their heat dissipation rate.
1. INTRODUCTION.
The use of polymeric solid matrices as an alternative to conventional liquid-solution dye lasers has been extensively studied [1]. The large volumes required, flammability, solvent evaporation, flow fluctuation and toxicity are some of the disadvantages of liquid-solution dye lasers that would be ameliorated by the use of polymers as solid matrices.
Solid polymer-glass solutions of rhodamine 6G, Rh6G, have been synthesized and their photophysical and lasing properties studied [2]. One of the main problems encountered upon the use of these solid matrices is the thermal- and/or photo-degradation of the material, tentatively explained in terms of the poor heat dissipation in the matrix [3-5]. Attempts to ameliorate this situation include the systematic variation of the polymer-matrix composition and the covalent binding of the dye to the polymeric chain, both actions aimed at increasing the heat dissipation rate [6-8].
In a previous study, laser-induced optoacoustic calorimetry was used to examine the thermoelastic properties of polymer matrices containing Rh6G [9]. A small difference in the adiabatic expansion coefficient of the polymers was detected and found to correlate with the long-term stability of the laser material. This suggests a key role of the heat dissipation processes in the lifetime of these polymeric materials.
In order to gain some insight into the heat dissipation processes we set out to determine the local thermal diffusion coefficient of the polymers. The time-resolved thermal lens technique (TRTL) was applied to this end. TRTL is especially suited for this purpose since it probes the dynamics of the lens induced locally by the heating processes, i.e. the refraction index changes occurring in the region illuminated by the pump laser beam [10]. We recognise that it is the local thermal diffusion coefficient, rather than the bulk one, which is relevant to the heat dissipation rate in the actual use of these polymeric solid laser dyes and make no a priori assumption as whether the two should be equal or not.
2. MATERIALS AND METHODS.
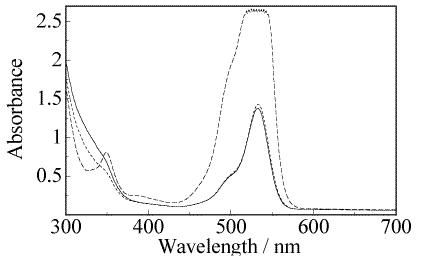
The matrices assayed were copolymers of methyl methacrylate (MMA) and 2-hydroxyethyl methacrylate (HEMA) (1:1 and 7:3 v/v) in which the dye was either dissolved or incorporated covalently bound to methacrylate through a benzylic ester linkage [2]. The preparation of the polymeric dye samples has been reported elsewhere [2,9]. The solid samples were cut to the appropriate shape (rods and disks) with front and back windows prepared by conventional grinding and polishing until optical-grade finish. Figure 1 shows a picture of the rods and disks prepared, as well as their absorption spectra.
|
 |
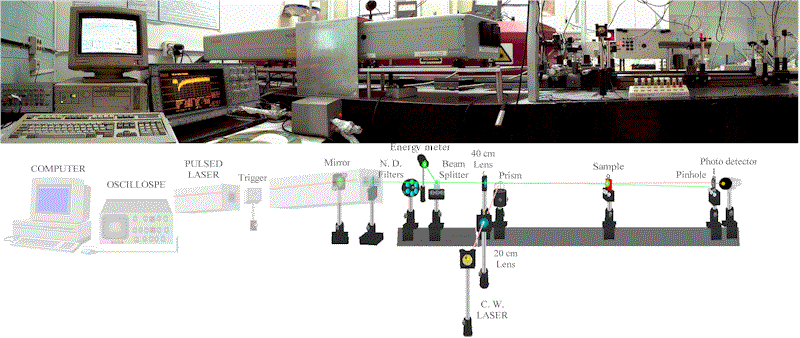
The set-up for the TRTL technique is shown in Figure 2 (actual picture and scheme). Samples were excited with the 2nd harmonic (532 nm) of a pulsed Nd:YAG laser (Continuum Surelite I) and the thermal lens was probed with a 5 mW CW diode laser at 650 nm.
3. DATA HANDLING
Under our experimental conditions, the time profile of the thermal lens signal is given by eq. 1 [11]:
|
(1) |
where I(t) is the value of the probe beam intensity at time t, I(¥ ) is its end value, and A and B are two parameters that are determined by non-linear fitting of eq. 1 to the data.
A
is a measure of the maximum thermal-lens amplitude, i.e. at time zero, and is proportional to the pump laser energy (in terms of amount of quanta; eq. 2). For the purpose of this work, ensuring that A increases linearly with the laser energy serves to rule out unwanted phenomena such as biphotonic absorption.
|
(2) |
B
in turn is proportional to the thermal diffusion coefficient and should be independent of the laser energy (eq. 3). The ultimate goal of this work is to determine its value.
|
(3) |
where w is the pump laser beam radius in the sample.
4. RESULTS AND DISCUSSION
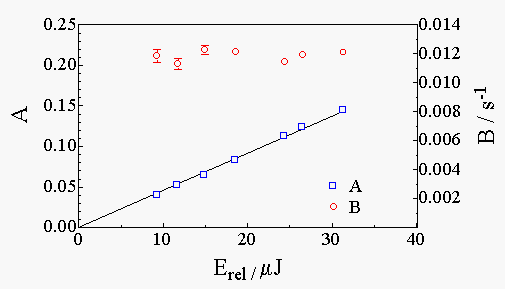
Figure 3
shows a typical TRTL experiment on Rh6G in water: the thermal lens signals at several laser energies, the residuals of the fit, and the laser energy dependence of the A and B parameters. Notice that A is proportional to the laser energy and B is essentially constant, as expected.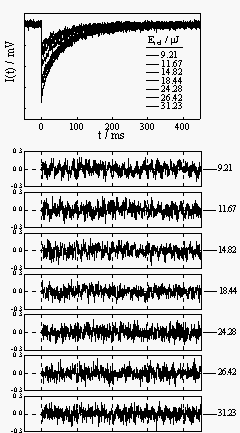 |
 |
 |
In Figure 4 the observed B values for a series of solvents are plotted against their reported thermal diffusion coefficients [12,13]. As expected, strict proportionality is observed, thus confirming the validity of the measurements. This plot will be used to determine the thermal diffusion coefficients of the polymers from their measured B values.
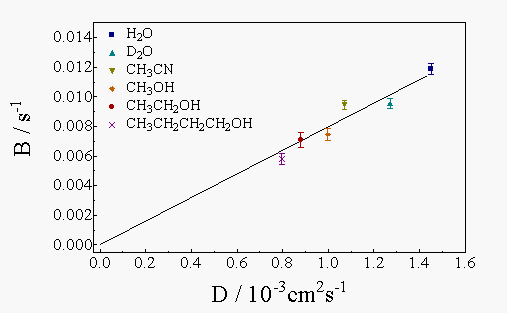
Figure 5 shows the effect of dye
concentration on the A and B parameters. The energy- The B parameter, in turn, shows a small but clear increase with Rh6G concentration, which probably reflects a corresponding increase in the thermal diffusion coefficient of the solution relative to the pure solvent. |
|
 |
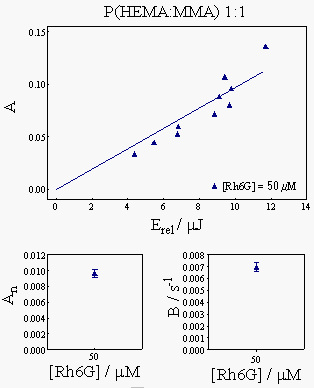
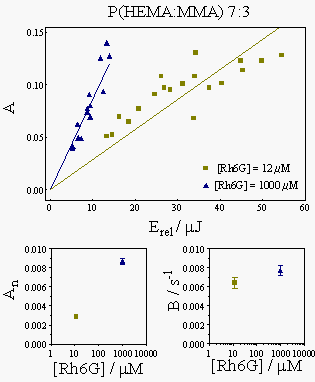
Figure 6 shows the actual results for the polymer samples, namely the dependence of the A and B parameters on the laser energy and on Rh6G concentration.
 |
 |
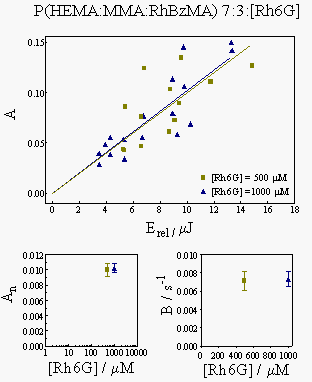 |
The measured B parameters were interpolated in the plot in Fig. 4 to yield the thermal diffusion coefficients. The results obtained are collected in the table below:
P(HEMA:MMA) 1:1 |
P(HEMA:MMA) 1:1 |
P(HEMA:MMA) 7:3 |
P(HEMA:MMA) 7:3 |
P(HEMA:MMA:RhBzMA) 7:3:500 µM |
P(HEMA:MMA:RhBzMA) 7:3:1000 µM |
|
[Rh6G] / µM |
12 |
50 |
12 |
1000 |
500 |
1000 |
D / 10-3 cm2 s-1 |
- |
0.87 ± 0.08 |
0.80 ± 0.10 |
0.97 ± 0.09 |
0.90 ± 0.16 |
0.92 ± 0.13 |
5. CONCLUSIONS
Analysis of the results leads to the following conclusions as to the thermal diffusion coefficients of the samples:
The observations 2, 3 and 4 lead to the general conclusion of this work, namely that the reported differences in the long-term thermal and photo-stability of the laser materials are not related to their heat dissipation rate
Acknowledgements
This work has been supported by the Spanish agency CYCIT through grants MAT-96-0654, MAT97-0705-C02-01 and PM98-0017-C02-2
.
REFERENCES
[1] A. Costela, I. García-Moreno, J.M. Figuera, F. Amat-Guerri.and R. Sastre: Laser Chem. 18, 63 (1998).
[2] F. López Arbeloa, T. López Arbeloa, I. López Arbeloa, A. Costela, I. García-Moreno, J.M. Figuera, F. Amat-Guerri.and R. Sastre: Appl.Phys.B 64, 651 (1997).
[3] I.P. Kaminow, L.W. Stulz, E.A. Chandross.and C.A. Pryde: Appl.Opt. 11, 1563 (1972).
[4] I.P. Kaminow, H.P. Weber.and E.A. Chandross: Appl.Phys.Lett 18, 497 (1971).
[5] R.L. Fork and Z. Kaplan: Appl.Phys.Lett 20, 472 (1972).
[6] F. Amat-Guerri, A. Costela, J.M. Figuera.and R. Sastre: Chem.Phys.Lett. 209, 352 (1993).
[7] A. Costela, F. Florido, I. García-Moreno, R. Duchowicz, F. Amat-Guerri, J.M. Figuera.and R. Sastre: Appl.Phys.B 60, 383 (1995).
[8] A. Costela, I. García-Moreno, J.M. Figuera, F. Amat-Guerri.and R. Sastre: Appl.Phys.Lett 68, 593 (1996).
[9] S. Nonell, C. Martí, I. García-Moreno, A. Costela, R. Sastre, Appl. Phys. B, (in press).
[10] M.C.Gupta, S.-D.Hong, A.Gupta.and J.Moacanin, Appl.Phys.Lett 37, 505 (1980).
[11] A.J.Twarowsky and D.S.Kliger, Chem.Phys. 20, 253 (1977).
[12] S.E.Braslavsky and K.Heihoff, in J.C.Scaiano (Editor), Handbook of Organic Photochemistry, p. 329, CRC Press, Boca Raton, Florida, (1989).
[13] C.V.Bindhu, S.S.Harilal, V.P.N.Nampoori.and C.P.G.Vallabhan, Opt.Eng. 37, 2791 (1998).
[14] D.W. van Krevelen, Properties of Polymers; Correlations with Chemical Structure, p. 235, Elsevier, Amsterdam (1972).