| Site home page | Conference home page | Discussion |
PHOTODYNAMIC INACTIVATION OF HERPES SIMPLEX VIRUSES WITH PORPHYRIN DERIVATIVES
Rodica-Mariana ION, Iolanda SAFTA
ZECASIN S.A., Photochemistry Department, Splaiul Independentei 202, Bucharest-79611, Romania; E-mail: irma@pcnet.ro
Giovanni NATILE
Dipartimento Farmaco-Chimico, Via E.Orabona 4, I-70125, Bari, Italy;
E-mail: natile@farmchim.uniba.it
ABSTRACT
The photodynamic inactivation of herpes simplex virus type I (HSV-1) provided from rabbit, hen, rat and bovine, by an anionic porphyrinic structures 5,10,15,20-tetra-sulfonato-phenyl porphyrin (TSPP) in PBS, has been studied in this paper. This porphyrin derivative demonstrates a remarkable virucidal activity upon light activation after 48 h. The concentration and temperature effects were evaluated, and also the time interval between dye treatment of cells and virus inoculation. A suitable membrane-photosensitizing dye is expected to inactivate HSV via the viral envelope, without damaging DNA which might unmask the oncogenic potential of the virus.
* The correspondence author
1. Introduction
The photodynamic therapy (PDT) of tumor is an innovative method for cancer diagnosis and treatment of various types of tumours by the combined action of oxygen, light and sensitizer [1].
Photodynamic inactivation has been known since Raab, at the beginning of the 20-th century, observed that acridine was harmless to paramecia in the dark and lethal when the organisms were exposed to visible light [2]. In 1958, Yamamoto [3] reported the first quantitative studies on photodynamic inactivation of bacterial virus. Neutral red was the first dye used in photodynamic inactivation of herpes [4]. Also, proflavine, methylene blue were used with good results for herpes simplex inactivation [5,6].
Herpes Simplex Virus (HSV) can be irreversibly and permanently made photosensitive by heterocyclic dyes so that brief exposure to visible light renders the virus noninfections [7].
Photodynamic inactivation is dependent upon the dye concentration, temperature and pH [8-10]. Membrane-photosensitizing dyes have the advantage of inactivating the virus at a site other than the genetic material [11]. A number of lipophilic and negatively-charged hydrophobic photosensitizers have been investigated for the cell killing both in vitro and in vivo. These include porphyrin derivatives [12]. and phthalocyanine derivatives [13]. Such complex systems have been tested in different culture cells killing [13,14]. The central and the meso-substituents are some important factors influencing the photodynamic activity having a photochemical yield for singlet oxygen generation [15].
2. Experimental part
2.1. Photosensitizers
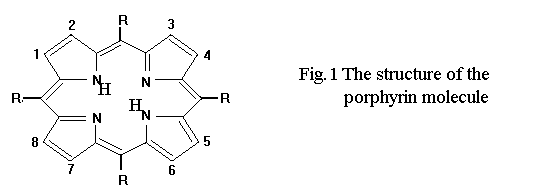
5,10,15,20-tetra-sulfonato-phenyl porphyrin (TSPP) , Figure 1, was synthesized and purified in the lab. after literature method [16].

R= -C6H4-SO3
The dye was stored at 4 oC as 0.44 x 10-4 M stock solutions in PBS and sterilized by filtration .
2.2. Viruses
All sera and derma tissue culture media were purchased from Merck, and the cell lines were grown with Bacto- Difco Normal Serum provided from the same firme.
2.3. Light exposure
The light source was a Hg-medium pressure lamp. For exposure of porphyrin-containing samples, the light was filtered by a cut-off filter (l> 650 nm).
The incident fluence rate was 200 W/m2. Exposure of viral particles and cells was done in 2 ml of phosphate buffered saline (PBS) containing 1.5 % fetal bovine serum (FTS) in 5 ml polystyrene plates at different temperature (35 C, 37.5 C, and 42 C) for 30 minutes, resulting in adose of 18 J/cm2.
2.4. Viral photoinactivation.
The cells were infected with 200 ml of cell-free viral suspension at a concentration of 2.6-2.9 x 105 (two suspension type A type with a cells concentration 2.6-2.8 x 105 per cell standard, and B type with a cells concentration of 2.7-2.9x105 per cell standard, for various time intervals at 37 C (and 30 % humidity in the first 5 days and 5 % CO2 in the last two days).The dye was added and the plates were exposed to light. After that, the plates were virused and overlaid with 2 % methylcellulose in Eagle’s minimal essential medium with non-essential amino acids (supplemented with 2% FCS). The plates were incubated at 37 C for 5 days in 30 % humidized incubator and in 5% CO2 humidified incubator in the last two days. Plaques were scored and the titers calculated based on the mean count of three replicate cells.
3. Results and discussion
Rate studies of photodynamic inactivation of viruses as a function of time at constant light intensity show like in figure 2, expressed by the survival ration (N/N0) plotted against time.
Figure 2 shows the survival curves of dermal HSV from rats during photosensitization with TSPP. Could be observed that only TSPP with 1.377 x 10-5 M concentration is the most efficient during the inactivation process of HSV. The other concentrations are not proper for this inactivation. This fact could be interpreted knowing different aggregated and ionized forms of TSPP [17-19]. and geometrical configurations of these forms with their photochemical activity [20].
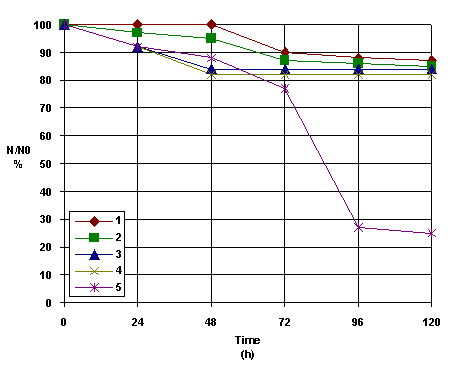
Figure 2. The survival curves of HSV -derma from rats at different TSPP concentrations
(T= 35 C) 1=0.274 x 10-4 M; 2= 0.6885 x 10-4 M; 3= 2.754 x 10-6 M; 4=5.508 x 10-6 M; 5= 1.377 x 10-5 M.
The aggregation processes of TSPP depend on porphyrin concentration [8], pH [10], ionic strength [21], temperature [9]. This porphyrin is an anionic one with a very large disk-shaped moloecule which possess four negative charges (the sulfonate groups from the four corners (Fig 1).
In aqueous solutions, at neutral pH, the electronic absorption spectrum of TSPP is typical for free base porphyrins (D2h symmetry) and is characterized by an intense Soret band at about 412 nm and four Q bands in the 500-700 nm range (the aetio-type spectrum). By increasing the medium acidity, complicated changes of the absorption spectrum occur for the TSPP aqueous solutions [10,22]. The Soret band is red shifted to 435 nm, while the Q bands become less intensive. New bands appear at 710 nm and 490 nm. The absorption intensity of these bands becomes dominant when the concentration of TSPP exceeds 10 -5M and they are attributed to the aggregated forms of dicationic species (c> 10-4M). The band from 490 nm arises from the J-aggregate (edge-to-edge interaction of porphyrin molecules [23].
TSPP have a zwitterionic structure between the double charge from the macrocycle inside (central dicationic group) and one of the other negative charges provided by the suphonic groups, all these being responsible for J-aggregation. So, in the studied case from this paper, TSPP at 1.377 x 10-5 M have a J-aggregated forms being a proof for the special efficient activity of this porphyrin. In other literature reports, similar data concluded that J-aggregates form due to their spiral geometric forms have a special ability to penetrate more efficient the cellular membrane [17,24]. After penetration of the cellular wall, the J-aggregation can be transformed into monomeric form, exhibiting a high efficiency for singlet oxygen generation.
The photosensitization reactions showed increases in the overall reaction rates with temperature. This suggests that the influence of hyperthermia on PDT may caused by in part to the temperature dependence of the basic chemical processes which play a role in photosensitization. The photosensitized HSV inactivation is presented in Figure 3 for three temperatures : 35, 37.5 and 42 C.
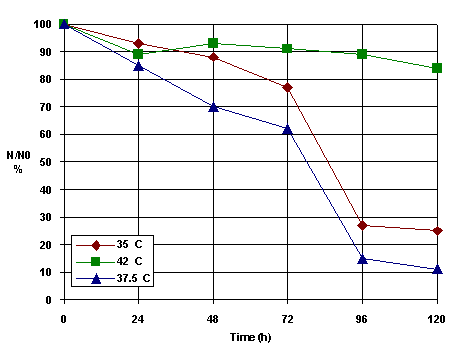
Figure 3. The HSV-derma from rats inactivation kinetics at different temperatures
It can be seen that the optimum value of temperature is 37.5 C because at this temperature TSPP could exist in the solution as a monomer and J-aggregates mixture in good agreement with other literature reports [25]. The photosensitization in vitro carried out under conditions of simultaneous hyperthermia (caused by heating of cell suspension) could involve a decrease of singlet oxygen quantum yield at high temperature [26]. The mechansim of photoinactivation of viruses is not completely undesrtood. Previous studies have shown that merocyanine dyes bind to the viral envelope and that photoexcitation leads to structural damage of the viral envelope [26].
By changing virus type (rats, hen, rabbit, bovine) could be observed that at the optimum temperature is observed a better efficiency for the suspensions with higher cell concentrations (2B), Figure 4.
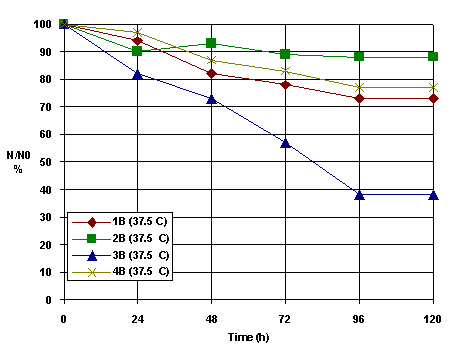
Figure 4. The inactivation kinetics for different HSV-derma cells (B-type suspension)
Generally speaking, three types of mechansim may be involved in the rapid response of tumours to PDT [27]. By comparison these results with those obtained for A type HSV derma cells, it was possible to observe that the rat derma HSV cells was more sensitive to this inactivation procedure (at 37.5 C) followed by hen, bovine and rabbit derma HSV cells (Figure 5).
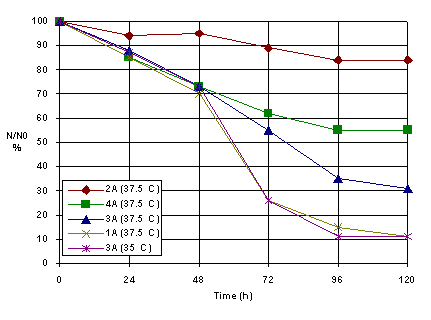
Figure 5. The HSV derma inactivation for different temperature (A-type cells suspension)
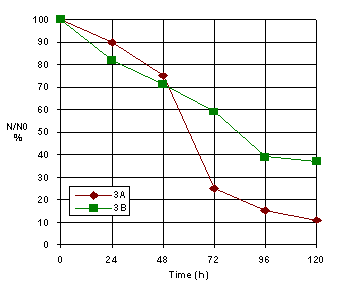
Figure 6. The inactivation kinetic for different cells-type (A and B)
The cell concentration from HSV suspension is essential for the inactivation procedure. The higher cells concentration , the shorter inactivation time (Figure 6).
The viral membrane or protein coat, might serve as a barrier to the penetration of the photosensitizing dye and the sensitivity of the virus perhaps is determined primarly by the permeability of its exterior layer or interface with the suspending medium..
The HSV envelope was found to be the major targets for the photodynamic damage following dye inactivation.
The main steps of photosensitization involved could be: dye partition into membrane; fluorescence quenching of bound dye; energy transfer experiments; viral adhesion to the host cells [26]
The sensitization with any of the photodynamically active dyes would be explained by assuming that the protein coat by this virus is virtually impermeable top dyes, or alternatively, that all of the important sites for combination of dye molecules with the RNA core of the virus are occupied or blocked by the peripheral protein.
At dye concentrations less than 10-4M and with prelonged exposure to intense light, degradation of guanosine occurred but adenosine, cytidine, and uridine were unaffected . Bond cleavage was assumed to have occurred at the points indicated in Fig.7 yielding ribosylurea, ribose, guanosine, and urea as the major end products.
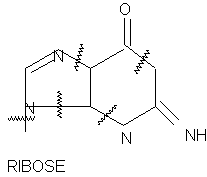
Figure 7.The photochemical degradation of nuclei acids involving the ribose structure
Some authors suggested that nucleic acids may be an important targets for PDT inactivation in their model viruses [33-35].
4. CONCLUSION
The photodynamic inactivation of herpes simplex virus type I (HSV-1) provided from rabbit, hen, rat and bovine, by an anionic porphyrinic structures 5,10,15,20-tetra-sulfonato-phenyl porphyrin (TSPP) in PBS, has been studied in this paper.
This porphyrin derivative demonstrates a remarkable virucidal activity upon light activation after 48 h, especially for HSV hen derma at 37.5 C The concentration and temperature effects were evaluated, and also the time interval between dye treatment of cells and virus inoculation .
5. REFERENCES
1. R.M.Ion, The photodynamic therapy of cancer-a photosensitisation or a photocatalytic process?,Progr.Catal.,1,55 (1997);
2. O.Raab, Ueber die Wirkung fluorescirender Stoffe auf Iufusorien, Z.Biol.(Munnich),39,524(1900);
3. N.Yamamoto, Photodynamic inactivation of bacteriophage and its inhibition, J.Bacteriol., 75, 443 (1958);
4. T.D.Felber, E.B.Smith, J.M.Knox, Photodynamic inactivation of herpes simplex, J.AMA, 223,289(1973);
5. C.Wallis, J.L.Melnick, Photodynamic inactivation of animal viruses: a review, Photochem.Photobiol., 4,159(1965);
6. M.I.Simon, V.Vunakis, The photodynamic reaction of methylene blue with DNA, J.Mol. Biol., 4,488(1962);
7. C.Wallis, J.L.Melnick, Irreversible photosensitization of viruses, Virusology, 23, 520 (1964);
8. R.M.Ion, G.A.Petre, The spectral study of the porphyrins effects of porphyrins and phtalocyanines, used in PDT.The aggregation processes, St.Cerc. Biotehnol., 29-30,122-127(1997);
9. R.M.Ion, M.Grigorescu, F.Scarlat, V.I.R.Niculescu, K.Gunaydin, Light, electron and photons beam effects on TSPP used in PDT, J.Balkan Union Oncology, 3(2)000(2000);
10. R.M.Ion, G.A.Petre, The spectral study of the porphyrins effects of porphyrins and phtalocyanines, used in PDT.The ionization processes, St.Cerc. Biotehnol., 29-30,113-121(1997);
11. W.Snipes, G.Keller, J.Woog, T.Vickroy, R.Deering and A.Keith, Inactivation of lipid-containing viruses by hydrophobic photosensitizers and near-UV radiation, Photochem.Photobiol., 29,785(1979);
12. R.M.Ion, A.Planner, K.Wiktorowicz, D.Frackowiak, Incorporation of various porphyrins into human blood cells measured using the flow-cytometry, the absorption and emission spectroscopy, Acta Biochimica Polonica, 45(30),833(1998);
13. R.M.Ion, I.Yilmaz, O.Bekaroglu, Supramolecular assemblies of pyrydil porphyrin and diazadithya phthalocyanine, Serb.J.Chem.Soc., 64(7-8)453-462(1999);
14. S.Agirtas, R.M.Ion, O.Bekaroglu, Spectral study of the supramolecular assemblies porphyrins-phthalocyanines for PDT, Mat.Sci. Eng.C:Biomimetic Materials Senzors Systems, C 396(1999);
15. a. R.M.Ion, A.Stirbet, The photodynamic action of some porphyrinic sensitizers on Escherichia Colli, St.Cerc.Biotehnol.,29-30,106-122(1997);
b R.M.Ion, Photochemical production and quenching of singlet oxygen by the porphyrins used in PDT, Rom. J. Biophys., 6(3-4), 205-212 (1996);
16. K.Berg, Cellular uptake and photosensitizing effects of porphyrins and phthalocyanines, Ph.D.Thesis, Oslo,Norway, 1992;
17. A.Planner, R.M.Ion, K.Wictorowicz, D.Frackowiak, The incorporation of porphyrins in human leucocytes measured by flow cytometry absorption and emission spectroscopy, "First Internet Conference on Photochem. Photobiol.", http://www.netsci-journal.com/97v3/intro.htm.
18. R.M.Ion,The photophysical properties of some porphyrins in binary mixtures of solvents,Rom.J.Biophys.,2,(1998);
19. a. R.M.Ion, M.Grigorescu, F.Scarlat, V.I.R.Niculescu, K.Gunaydin, Aggregation and degradation of TNP in radiation fields, Rom.Reports Phys., 2(1999);
b. R.M.Ion, M.Grigorescu, F.Scarlat, V.I.R.Niculescu, K.Gunaydin,Radiation processed Hp for combined PDT, EOCC-10, Vienna, 1999;
20. R.M.Ion, Spectral analysis of the porphyrin into human blood cells, J.Biomed.Opt., 4(3),319(1999);
21. K.Kemnitz, T.Sakaguchi, Water-soluble porphyrin monomer-dimer systems:fluorescence dynamics and thermodynamic properties, Chem.Phys.Lett., 196,497(1992);
22. G.J.Smith,K.P.Ghiggino,L.E.Bennett,T.L.Nero,The "Q-band" absorption spetra o hematoporphyrin monomer and aggregate in aqueous solution, Photochem. Photobiol.,49,49-52(1989);
23. J.M.Ribo, J.Crusats, J.-A.Farrera, M.L.Valero, Aggregation in water solutions of tetrasodium diprotonated meso-tetrakis (4-sulfonatophenyl)porphyrin, J.Chem.Soc.Chem.Comm., 681(1974);
24. R.M.Ion, Spectral studies of TSPP and TSNP used in PDT.I.Monomer-dimer equilibrium, Rom. J. Biophys., 6(3-4), 213-218 (1996);
25. D.Frackowiak, A.Planner, R.M.Ion, K.Wiktorowicz, Incorporation of
dyes in resting and stimulated leukocytes,in "Synthesis, properties and applications of near-infrared dyes in high technology fields", Ed.S.Daehne, Kluwer Acad.Publ., NATO ASI Series,1998;
26. a.J.M.O’Brien, D.K.Gaffney, T.P.Wang, F.Sieber, Merocyanine 540-sensitized photoinactivation of enveloped viruses in blood products: site and mechanism phototoxicity, Blood, 80,277(1992);
b. V.Gottfried, S.Kimmel, Temperature effects on photosensitized processes, J.Photochem.Photobiol.B:Biol., 8,419-430(1991);
27. M.Ochsner, Photophysical and photobiological processes in the photodynamic therapy of tumours, J.Photochem.Photobiol.,B:Biol.,39,1-18(1997);
28. L.Danaila,M.L.Pascu,R.M.Ion,L.Tugulea,The photodynamic therapy of brain tumours, EMLA Symp.,1998,Bucharest;
29. L.Danaila,M.L.Pascu,A.Popescu, R.M.Ion, Spectrophotometric chracterization of useful dyes in laser photodynamic therapy, Vol SPIE, 4068, 712 (2000);
30. M.L.Pascu,L.Danaila, R.M.Ion,Researches containing the applications of laser photodynamic therapy in neurosurgery, Rom.Rep.Phys., accepted 1999;
31. M.L.Pascu, A.Popescu, N.Carp, R.M.Ion, M.Pascu, Photodynamic therapy studies on brain tumors using nitrogen pulsed lasers, Proc.SPIE, 4166,140(2000);
32. a. R.M.Ion, M.Grigorescu, F.Scarlat, V.I.R.Niculescu, K.Gunaydin, The type and intensity radiation effects on porphyrins used in PDT, Int.Conf.Molecular and Izotope Processes, Cluj, 1999;
b. R.M.Ion, Combined treatment methods for cancer therapy, Invited conference Antakya-Hatay, Turkey, 1999;
33. C.W.Hiatt, Inactivation of viruses by photodynamic action, Studia Biophysica, 3,157(1967);
34. Z.Smetana, E.Ben-Hur, E.Mendelson, S.Salzburg, P.Wagner, Z.Malik, HSV proteins are damaged following photodynamic inactivation with phthalocyanines, J.Photochem.Photobiol.,B:Biol., 44,77(1998);
35. R.M.Ion, K.Gunaydin, The effects of photodynamic action on cellular targets.II.The interaction between cationic dyes and azotate bases, St.Cerc.Biotehnol.,(Bucharest), 30-32, 216(1998);
36. R.M.Ion, K.Gunaydin, The effects of photodynamic action on cellular targets.II.The interaction between cationic dyes and DNA, St.Cerc.Biotehnol.,(Bucharest), 30-32, 237(1998);