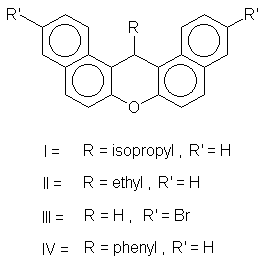
| Site home page | Conference home page | Discussion |
THE SPECTROSCOPY AND ASSOCIATED PHOTODYNAMIC ACTIVITY OF SOME 14-ALKYL-14H-DIBENZO[a,j]XANTHENE DERIVATIVES
Rodica-Mariana Ion [a] , Carmen Albulescu[a] , Okan Sirkecioglu [b] and Naciye Talınlı [b]
[a] ZECASIN S.A., Photochemistry Dept., Splaiul Independentei 202, Bucharest- 79611,
Romania; E-mail: irma@pcnet.ro
[b] Istanbul Technical University, Faculty of Science, Dept. of Chemistry, 80626-Maslak,
Istanbul-Turkey.
Keywords: Photodynamic activity, dibenzo [a,j] xanthene, antitumorial agent.
Abstract
The absorption properties ( absorption bands and molar absorption coefficients) and the quantum yields for singlet oxygen generation for different substituted dibenzo[a,j] xanthene derivatives (R= isopropyl, ethyl, phenyl, hydrogen and R1=bromine substituents) were evaluated for such compounds with potential applications as photodynamics drugs. Saccharomiecs Cerevisiac cells were used as an experimental model for studying the association of photodynamic therapy (PDT) with antitumorial agents ( 14-isopropyl-14H- dibenzo [a,j] xanthene ) and with ionizing radiation (electron beam - 800R). The cultures were incubated 18 hours at 37°C with the drug, trypsinized and exposed to high pressure mercury lamp. The obtained results showed that combined application of PDT with dibenzoxanthene and ionizing radiation strongly increases the reduced the inhibition of PDT caused by acute hypoxia.
1. Introduction
The xanthene derivatives constitute intermediates in synthesis and have interesting applications for laser technologies due to their useful spectroscopic properties[1,2] . The different substituent on the molecule can modify the symmetry of the radiative transition. These compounds are polarized along the 14a and 13b (far to the oxygen atom) of the dibenzo [a,j] xanthene molecule.
Comparing xanthene compounds by analogy to the triphenylmethane dyes, they can be similar as a dimensional chromophore system in which the X-band is oriented along the long axis passing the 14a and 13b positions, corresponding to the longest wavelength absorption and the Y-band absorption to the shorter wavelength.
The absorption properties (absorption bands and molar absorption coefficients) and the quantum yields for singlet oxygen generation for different substituted dibenzo [a,j] xanthene derivatives (R= isopropyl, ethyl, phenyl, hydrogen and R1= bromine substituents) were evaluated for such compounds in order to establish the influence of the substituents on their molecular structure.
Photodynamic therapy (PDT) is a method of treating tumours by the combined use of a photosensitizer and light. PDT uses the light of a specific wavelength to excite a preadministrated photosensitizer drug, for localized tumor control[3,4] . It has been demonstrated that such treatment will cause both direct PDT cytotoxicity and tissue injury associated with local microvasculature damage[5,6] . Tumor methabolic status and blood perfusion have been shown to decrease immediately after a PDT treatment[7] . Singlet oxygen has been implicated as agent causing injury to cells and tissues[8] . In the literature many efforts could be met in order to discover new photosensitizer with high singlet oxygen efficiency.
The xanthene derivatives are reported for the first time as new sensitizers for such application area. Irradiation of an aqueous suspension of living cells by ionizing radiations causes a series of complicated reactions involving ionizations and excitations of water inside and outside of the cells [9] . Accumulated evidence indicates that hydrogen and hydroxy radicals and solvated electrons are the primary species responsible for most of these effects [10]. Modern cancer treatment is often a combination of different modalities of treatment. PDT has been successfully combined with ionizing radiation and chemotherapy using different antitumorial drugs which are known to potentiate cellular damage in hypotoxic tumors treated with ionizing radiation.
This paper will deal with the photodynamic activity of xanthene derivatives on SC suspended cells combined with electron-800R- in order to evaluate new type of sensitizer for this area.
2.Experimental Part
The xanthene derivatives were synthesized and purified as reported earlier [1]. Their structures are shown in Figure 1.
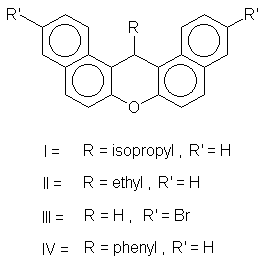
Figure 1. The structure of the the dibenzo [a,j] xanthene molecule.
Benzene as non-polar solvent (Merck) was used as received.
The UV-Vis spectra were obtained using a SPECORD M 400, Carl Zeiss Jena apparatus. The quantum yield of singlet oxygen generation was determined using 1,3-diphenylisobenzofuran (DPBF) test [3].
The singlet oxygen trap, DPBF, obtained from Fluka (Purum grade) was used as received.
DPBF test for singlet oxygen:
Measurements were carried out in a quartz cell (1cm x 1cm) at 20C. A benzene solution (2.3 ml) containing sensitizer (4.8 x 10-5 M) and DPBF (2.7 x 10-5 M) was irradiated with beam of a UV-VIS spectrophotometer with benzene as reference. Solutions of sensitizers were freshly prepared and kept in the dark before measurements. The decreasing DPBF concentration was followed by a special programme ruled on the computer measuring the absorbance at 415 nm (molar absorption coefficient= 23300 M-1 .cm-1) as function of the irradiation time (irradiation cycles 50 x 25 s). The reaction showed zero order-kinetic in the first 100s. The incident photon flow was 4.65 x 10-9 Ms-1. Using the absorbtion spectra of the sensitizers the absorbed photon flow ( I abs) was evaluated. Quantum yield of the photo-oxidation of DPBF were calculated using ;
F[DPBF]= (d[DPBF]/dt) / ( I abs/V)
1/F[DPBF]= 1/F(1O2) + 1/F(1O2).kd/ka.1/[DPBF]
From the intercept of the plot 1/F[DPBF] vs.1/[DPBF] could be obtained F 1O2.
The singlet lifetime (radiative lifetime) of the xanthene derivatives was calculated with an integration method, ruled on an ATARI computer. The formula used was that given by Bowen and Wokes [4].
1/tr - 2900 n2 no2 e dn (tr in seconds) (1)
where e dn is the area under the curve of molar absorption coefficient plotted against wavenumber ;
no is the wavenumber of the maximum of the absorbtion band ;
n is the refractive index of the solvent.
The cells were collected at the same periods of growth in liquid yeast extract-peptone medium containing 0.1 % dextrose, washed and resuspended in water solution. Yeast cells are advantageous to many organisms since they may be suspended in distilled water without detectable damage, an ideal condition for avoiding the possible supplimentary reactions involving buffers or other additives.
Irradiation with ionizing radiation
The electron and photon radiation beams were supplied by a 7.0 Mv Linear Accelerator of the National Institute for Laser, Plasma and Radiation Physics, Romania. Their main parameters were as follows [5]: average power Pav=56 W; average current Iav= 8 mA; pulse duration t=4 ms; frequency f= 100 Hz; distance between window and irradiation place L=70 cm.
Irradiation with light
All the irradiation processes with light were carried out with a medium-pressure 1000 W mercury lamp (Romlux-Romania).
3. Results and Discussion
The xanthene derivatives like triphenylmethane dyes reported for the first time in the literature as sensitizer, was characterized and tested in this paper as therapeutic compounds on Saccharomices Cerevisiae culture cells- in vitro-. Both light irradiation and ionizing irradiation were applied on the same culture cells. Testing the photodynamic activity of such xanthene compounds on SC culture cells was observed that the survival curves of SC were exponential for stationary phase cells irradiated with monochromatic light (650 nm). The ionizing radiation was capable to strongly decrease the survival fraction of such culture cells , so increasing the photodynamic activity.
The obtained results showed that combined application of PDT with dibenzoxanthene and ionizing radiation strongly increases the application area of these compounds.
Table 1 gives the position of the wavelength absorption band for the series xanthene salts at room temperature. The long wavelength absorption band is polarized along the x-axis and is attributed to a p® p* transition [11]. The absorption bands from 200 and 340 nm could be attributed to the polarized transition.
Table 1. Position of the wavelength absorption band in benzene solution.
|
Sample No. |
l/e (nm/M-1 cm-1) |
|
I |
321/18640 333/18510 420/1280 |
|
II |
322/13090 334/12780 420/270 |
|
III |
305/33165 330/14924 346/12408 |
|
IV |
320/10740 335/10120 |
As expected [12] the substituted group (para substitution to the 14- position of dibenzoxanthene group) induces a slight red shift of the absorption band indipendent of the electron-donating properties of the substituent. The substituent effect is more selective when it involves the 14a- ( or 13b-) position of the pyranoyl ring of dibenzoxanthene along the polarization axis. Substitution to 14a- or 13b- on phenyl group along the polarization axis by electron-donating inductive effects (Br) as in (III) induces a blue shift of the absorption band while substitution with electron-donating groups in the position 14- (alkyl) slight shifts the absorption to the red; the effects are not additives, as demonstrated by compound III which bears both types of substituents. More interesting is the increase in rigidity of the phenyl substituents on blocking their rotation by incorporation them in a six-membered ring , resulting in a strong red shift of the 333 nm band by 13 nm ( compare sample I with III). But blocking the phenyl group at the 14-position of the pyranoyl ring does not produce any important effect since it is not oriented along the polarization axis.
The xanthene compound (III) can be modified by electron beam (in good agreement with the literature report, [10]) being transformed in acidic and secondly in dimeric form, as could be observed by the dissapeareance of almost of the absorption bands .
DPBF photooxidation can be sensitized by all studied compounds. The xanthene- sensitized generation of singlet oxygen are shown in table 2.
Table 2. The singlet oxygen quantum yields and radiative lifetimes
|
Compounds |
Condition |
F [1O2] |
t x 109 (s) |
|
III |
200 R/e |
0.168 |
0.05 |
|
III |
200 R/e |
0.78 |
0.095 |
|
I |
0.75 |
0.12 |
|
|
I |
200 R/e |
0.96 |
0.19 |
|
II |
0.38 |
0.54 |
|
|
IV |
0.66 |
0.22 |
Testing the photodynamic activity of the best xanthene compoud (I) was observed that the survival curves of SC only were exponential for stationary phase cells irradiated with 650 nm. The genotoxic and carcinogenetic potential of ionizing radiation has been thoroughly investigated. And it is well-known that radiation is capable of inactivating yeast cells with dose dependencies different [13], suggestion that an additional type of lesions are induced by radiation (Fig.2).
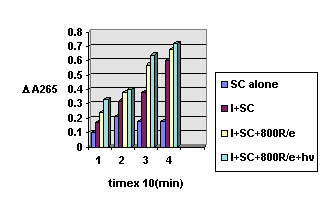
Figure 2. The hystogram of SC culture cells during the combined photodynamic treatment
These additional lesions presumably lead to the well-characterized increases in nuclear gene mutation frequency caused by radiation. If DNA strand break (single) is the major damage, as in case of X-ray, g-ray irradiation [11] in the PDT, the singlet oxygen is the major reactive intermediate. The primary site of oxygenation in DNA would locate in the base residues. This might cause a strong decrease of 265 nm absorbance value (due to the proteic aminoacids and DNA-purine and pyrimidine bases).
Thus, in spite of singlet oxygen generation, some common responses to ionizing radiation could occur, PDT being unique in the subcellular localization of damage. The dose-response curve for this case is logarithmic as expected in the case of ionizing radiation. Since the singlet oxygen does not travel more than 0.1m during its lifetime, part of the DNA in the middle of the nucleus may remain unattacked during the treatment. The dose-response curve for the sensitizer bends downwards in the semi-logarithmic plot probably because the radiation causes deaggregation or a relocalization of the sensitizer in the cells. Meanwhile the change of the xanthene compounds during the radiation treatment could be observed in Fig 3.
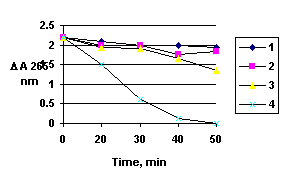
Figure 3. The time-dependence of DA 265 nm (coresponding to the proteic part of SC) during ionizing radiation
1- SC alone; 2- SC+sample I +800 R/e; 3- SC+Sample I +800R/e; 4-SC+ sample I +800R/e +hn
In the studied case, at the same survival level PDT with sensitizer induces a similar or a larger amount of DNA single-strand breaks than do g-rays.
4. Conclusion
The absorption properties ( absorption bands and molar absorption coefficients) and the quantum yields for singlet oxygen generation for different substituted dibenzo[a,j] xanthene derivatives (R= isopropyl, ethyl, phenyl, hydrogen and R1=bromine substituents) were evaluated for such compounds with potential applications as photodynamics drugs. Saccharomiecs Cerevisiac cells were used as an experimental model for studying the association of photodynamic therapy (PDT) with antitumorial agents ( 14-isopropyl-14H- dibenzo [a,j] xanthene ) and with ionizing radiation (electron beam - 800R). The obtained results showed that combined application of PDT with dibenzoxanthene and ionizing radiation strongly increases the reduced the inhibition of PDT caused by acute hypoxia.
5.References
1. O.Sirkecioglu, N.Talinli, A.Akar, J.Chem.Res.,(S), 502(1995);
2. R.M.Ion, V.L.Fara, Proc.Indian Acad.Sci., 107(6),825-830(1995);
3. R.M.Ion, PDT- a photosensitization or a photocatalytic process, Progr.Catal.,2,55-76(1997);
4. R.M.Ion, D.Frackowiak, A.Planner, K.Wiktorowicz, The incorporation of various porphyrins into human blood cells, Acta Biochim.Pol.,45(3)833-845(1998);
5. L.Danaila, M.L.Pascu, A.Popescu, M.Pascu, R.M.Ion, Researches concerning the application of laser PDT in neorosurgery, Rom.Rep.Physics, 1999, submitted;
6. R.M.Ion, Spectral analysis of the porphyrins incorporation into human blood cells, J.Biomed.Optics,4(3),1999;
7. Q.Chen, H.Chen, F.W.Hetzel, Tumour oxygenation changes post-photodynamic therapy, Photochem.Photobiol.,63(1),1228-31(1996);
8. W.C.Eisenberg, K.Taylor, R.R.Guerrero, Cytogenetic effects of singlet oxygen, J.Photo chem. Photobiol.,B:Biol.,16(1992)381-384;
9. T.Ito, K.Kobayashi, Induction of lethal and genetic damage by vacuum-UV irradiation of aqueous suspensions of yeast cells, Rad.res.,68,275-283(1976);
10. F.Y.Zhao, K.H.Zhang, H.N.Huang, K.H.Sun, Q.B.Ling, B.Xu, Use of HP as a sensitizer for radiotherapy of oral and maxillifacial tumours:a preliminary report, Lasers Med.Sci., 1,253-256(1986);
11. J.R.Wilt, G.Reynolds and J.A.Van Allan, Tetr.29,795(1973);
12. H.Khedija, H.Strzelecka and M.Simalty, Bull.Soc.Chim.Fr.,1398,3173(1972);
13. M.K.Cheng, J.McKean,D.Boisvert, J.Tulip, Misoimidazole combined with photo dynamic therapy of rat 9L gliosarcoma tumours, Lasers in Med.Sci.,4,79-84(1989);
14. C.A.Beam,B.K.Mortimer, R.G.Wolfe, C.A.Tobias, The relation of radioresistance to budding in Saccharomices Cerevisiae, Arch.Biochem.Biophys.49,110-122(1954);
15. T.Ito, Cellular and subcellular mechanisms of PDT action, Photochem.Photobiol., 28,493-508(1978);