| Site home page | Conference home page | Discussion |
Peculiarities of Water-Porphyrin Interaction in Large-Scale Porphyrin Aggregates
Alexander V. Udal’tsov1, Vladimir A. Sinani2, Lev A. Kazarin2, Alexei A. Sweshnikov2
Faculty of Biology1, Faculty of Chemistry2,
Lomonosov Moscow State University,
119899 Moscow, Russia; e-mail: audaltsov@mail.ru
The finding of large-scale porphyrin aggregates has been reported recently [1]. The formation of these aggregates was proposed to be due to strong involving of water into interaction with porphyrin. In this work properties of protonated meso-tetraphenylporphine (TPP) aggregates are investigated by absorption, circular dichroism and infrared spectroscopies. The protonated TPP aggregates were tested by scanning electron microscopy in thin films prepared by evaporation of different water-organic solutions. The aggregates with the sizes approximately from one to several micrometers are observed in the films. Two maxima at 388 ± 3 nm and 465 ± 3 nm are revealed in the CD spectra of protonated TPP in water-tetrahydrofuran solutions in the presence of 0.4 N hydrochloric acid. The second maximum coincides exactly with the corresponding maximum in the absorption spectrum. The both maxima in the CD spectra undergo some changes with an increase of porphyrin concentration. The changes can be associated with the process of aggregation or/and a photoreaction observed earlier [2]. According to the IR spectra, all water molecules are strongly bound in the porphyrin aggregates and involved in donor-acceptor interactions with porphyrin molecules and other water molecules forming a continuos microphase. The bands of valence vibrations of water as those of the deformational vibrations form the corresponding doublets at 3512, 3457 cm-1 and 1610, 1632 cm-1, respectively. A new broad band with the maximum located within 2136-2152 cm-1 region are revealed in the IR spectra of protonated TPP aggregates in the films, but this band is absent in the spectrum of meso-tetra(p-aminophenyl)porphine associated in thin film. These peculiarities are due to water-porphyrin interactions in the aggregates when the whole spatial structure of liquid water is involved in the interactions to delocalize the positive charges in TPP dimers.

Results It was interesting to study spectral characteristics of protonated forms of meso-tetraphenylporphine (TPP), which can aggregate in water-organic solutions [1], in comparison with meso-tetra(p-aminophenyl)porphine (TAPP) associated in solutions to find resemblance and
difference in the interaction of water with the porphyrins.
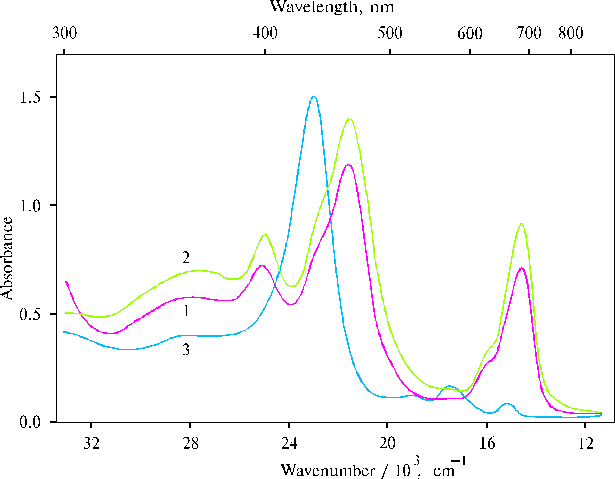
Fig. 1. Absorption spectra of TPP at a high concentration of porphyrin in the presence of 0.4 N hydrochloric acid in water-acetone solution (93 : 7, v/v), 1; and in water-dioxane solution (93 : 7, v/v), 2; and TAPP in dimethylformamide (DMFA) containing water traces, 3.
The spectra (Fig. 1, curves 1 and 2) exhibit strong and medium bands at 403, 465 and 694 nm (± 2 nm), while the spectrum of TAPP (curve 3) has one strong Soret band at 435 nm. Very minute particles of porphyrin aggregates can be seen in the first two solutions at a high concentration of porphyrin. But this green precipitate is completely dissolved under the mixing of the solutions although the presence of the strong opalescence indicate the aggregates in these solutions.
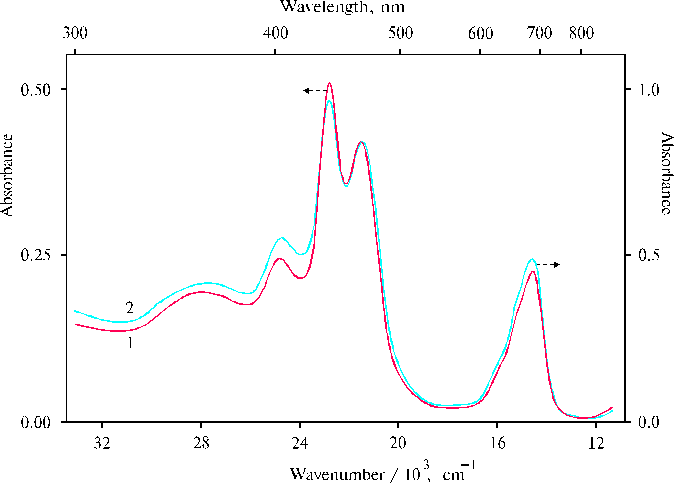
Fig. 2. Absorption spectra of TPP at a low concentration of porphyrin in the presence of 0.4 N hydrochloric acid in water-tetrahydrofuran solution (97 : 3, v/v), 1; and at twofold concentration of porphyrin in similar solution (94 : 6, v/v), 2.
At a low concentration of porphyrin, the spectra exhibit the new band at 437 nm which is observed as a shoulder in the previous spectra (Fig. 1, curves 1 and 2). Unfortunately, we can not tell for the present why this band is decreased under the aggregation of porphyrin. Although, the situation can be some elucidated from the next results.
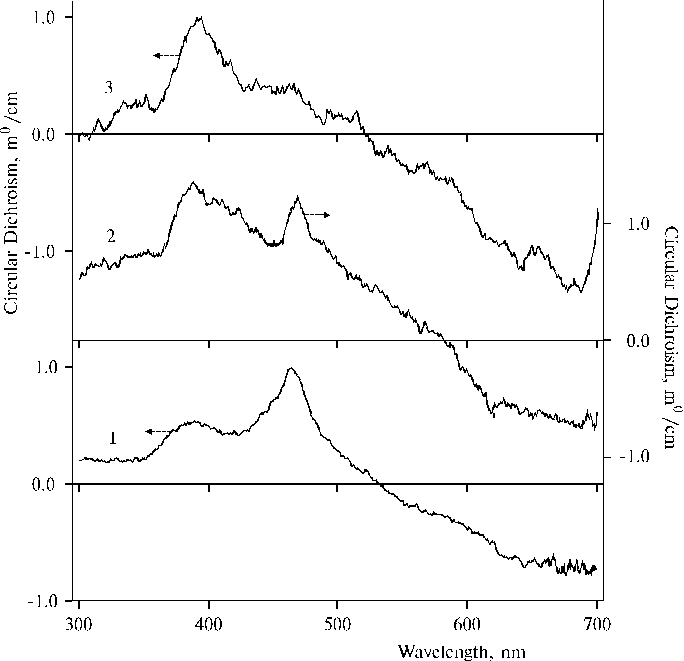
Fig. 3. Circular dichroism spectra of TPP in the presence of 0.4 N hydrochloric acid in water-tetrahydrofuran solutions, 1; and at twofold concentration of porphyrin, 2 (the experimental conditions are the same as indicated in the caption of Fig. 2); and TAPP in dimethylformamide containing water traces, 3.
The spectrum (Fig. 3, curve 1) exhibits two maxima, one of them at 465 ± 3 nm coincides with the corresponding maximum in the absorption spectrum (see Fig. 2) but the other maximum is located at 388 ± 3 nm. The latter maximum is increased and broadened in the spectrum (Fig. 3, curve 2) at a larger porphyrin concentration although the location of the maximum is not changed. At the same time the former maximum is red shifted by 5 nm and decreased in the spectrum (curve 2) as compared to the spectrum (curve 1). Some final state of this tendency of the changes is apparently observed in the spectrum of TAPP associated in DMFA (curve 3), where only a large band with the maximum at 395 ± 3 nm is present. The changes observed in the spectra (curves 1 and 2) can be associated with the aggregation of porphyrin at a larger porphyrin concentration or a photoreaction between different dimers in the porphyrin aggregates as the fluorescence and EPR spectra evidenced [1-2]. Hence, these results partially explain the changes in absorption spectra since a decrease of the 437 nm band is accompanied by an increase of the 388 nm band in the corresponding CD spectrum. But now we can not assign the observed changes in the absorption and CD spectra to only porphyrin aggregation or the photoreaction.
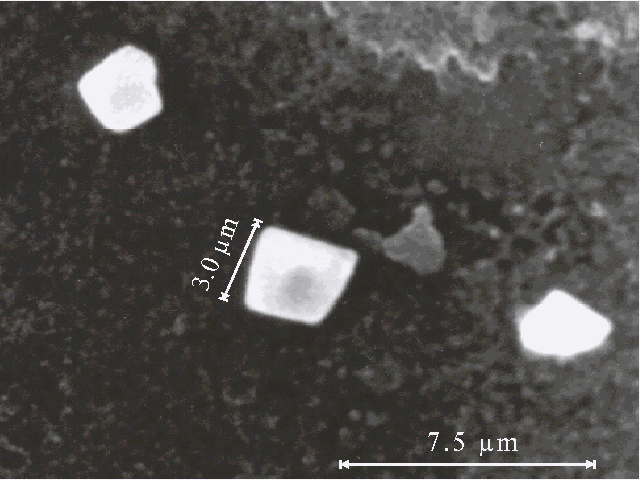
Fig. 4A. Microphotograph of TPP aggregates obtained on a glass plate directly after evaporation of water-acetone solution (93 : 7, v/v) in the presence of 0.4 N hydrochloric acid.
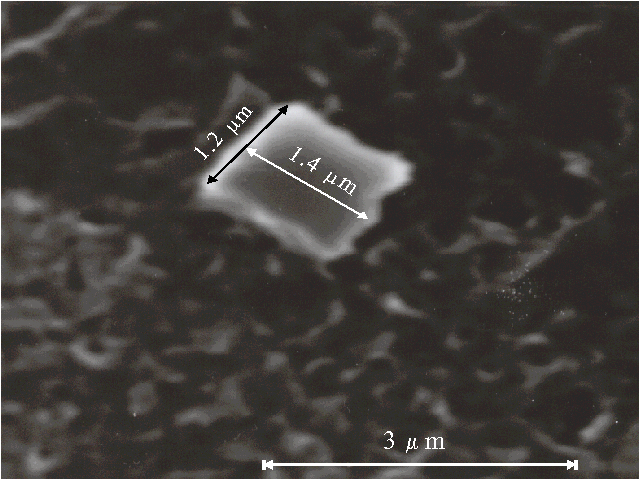
Fig. 4B. Microphotograph of TPP aggregate obtained on a glass plate directly after evaporation of water-dioxane solution (93 : 7, v/v) in the presence of 0.4 N hydrochloric acid.
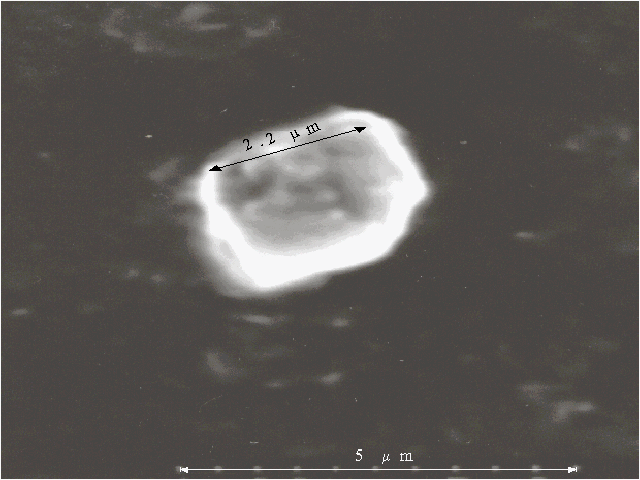
Fig. 4C. Microphotograph of TPP aggregate obtained on a glass plate directly after evaporation of water-tetrahydrofuran solution (93 : 7, v/v) in the presence of 0.4 N hydrochloric acid.
Porphyrin aggregates can be seen by scanning electron microscopy, the corresponding microphotographs are presented in Fig. 4 (A, B, C). We were looking for the separate aggregates to examine their sizes and surface since a picture of many aggregates often meets difficulties for the interpretation. The different bars of regular or irregular shape with the sizes of one-several micrometers are displayed on the microphotographs. It should be noted that no aggregates are observed by scanning electron microscopy in the absence of porphyrin in similar solutions.
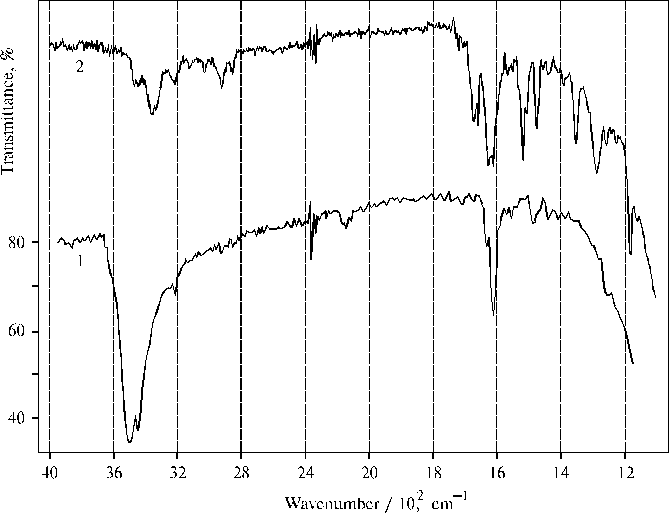
Fig. 5. Infrared spectra of TPP aggregates obtained on a CaF2 plate after evaporation of water-acetone solution (93 : 7, v/v) in the presence of 0.4 N hydrochloric acid, 1 (green color of the film indicate protonated state of porphyrin); and TAPP associated in thin film prepared on a CaF2 plate by evaporation of DMFA, 2.
It was interesting to compare IR spectra of protonated TPP forms and associated TAPP presented in Fig. 5. Only the bands belonging to water and some bands belonging to the porphyrin, where the 1484 cm-1 band is the most intense among the porphyrin bands, are mainly observed in the IR spectrum of the former. It is important to note that the frequency of valence vibrations (nN-H) in pyrrole rings of protonated TPP and associated TAPP coincides and equals 3220 cm-1. This suggests similar interaction between porphyrin and water involved in the solvate cover for the both porphyrins. The doublet of deformational vibrations of water (the strong 1610 cm-1 and weak 1632 cm-1 band) suggests all water molecules are bound inside the porphyrin aggregates and a major part of the bound water is involved as a proton acceptor, since the frequency is close to 1608 cm-1 when water molecules are proton acceptors under interactions [3]. Usually a water molecule interacting as a proton donor has the frequency of 1618 cm-1. But in the case of protonated TPP, the weak band of the doublet is located between normal deformational vibrations (1645 ± 5 cm-1) and the frequency of water molecules with proton-donor interactions (1618 cm-1).
Hence, water-porphyrin interaction in large-scale porphyrin aggregates has a pronounced donor-acceptor character and this interaction is directed to weaken the charge of the protonated porphyrin in the system. The deviation for the proton-acceptor interactions is 2 cm-1 but the deviation for the corresponding proton-donor interactions in the aggregates equals 14 cm-1. On the other hand, these results suggest that the system of tetrahedral network of hydrogen bonds of the liquid water is proved very flexible, so that a proton relaxation in the whole system is produced to delocalize the positive charge on porphyrin dimers. These peculiarities make the porphyrin aggregates quite stable but the water molecules in the aggregates are actually proved in some activated state. According to the deformational vibrations, a strong 3512 cm-1 band of the valence vibrations is responsible for the proton-acceptor interactions while a lesser 3457 cm-1 band belongs to water molecules with a proton-donor interactions in the system. It is very interesting that the IR spectrum of water activated by the interaction with protonated TPP in the aggregates (curve 1), exhibits a broad 2136 cm-1 band. This band can be assigned only to water but not porphyrin, since the concentration of the pigment in the aggregates is very low (see the vibrations of C-H bonds located at 2858 and 2928 cm-1 and some other porphyrin bands including the most intense 1484 cm-1 band). But no band similar to the 2136 cm-1 band is observed in the IR spectrum of TAPP associated in thin film (curve 2), when water is involved in the corresponding solvate cover but can not form any continuos microphase since concentration of water is low as compared to the concentration of the porphyrin and because of a quite large concentration of the organic solvent in the thin film (cp. the 1670 cm-1 band of DMFA and the 1653 cm-1 band of water). Details of water-porphyrin interaction in thin films of the aminoporphyrins are reported earlier [4].
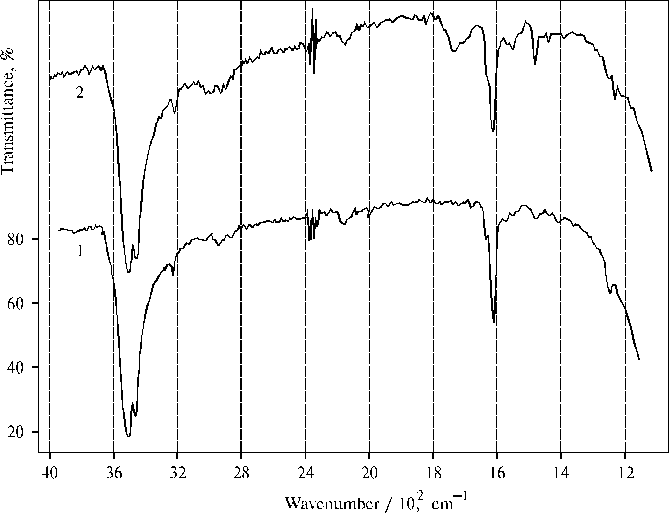
Fig. 6. Infrared spectra of TPP aggregates obtained on a CaF2 plate after evaporation of water-tetrahydrofuran solution (93 : 7, v/v) in the presence of 0.4 N hydrochloric acid, 1; and water-dioxane solution (93 : 7, v/v) in the presence of 0.4 N hydrochloric acid, 2 (green color of the films indicate protonated state of porphyrin). Similar characteristics of water-porphyrin interaction are observed in the IR spectra of protonated TPP in the thin films (Fig. 6) including the broad 2146 cm-1 and 2152 cm-1 bands for the films prepared by evaporation of water-tetrahydrofuran and water-dioxane solutions, respectively. The former IR spectrum is very similar to the spectrum presented in the previous figure (Fig. 5, curve 1). But the latter has some new bands (Fig. 6, curve 2). One of them is located at 1740 cm-1 that is apparently due to donor-acceptor interaction between water and porphyrin in the presence of minor amount of dioxane (boiling-point of 101o C), which has a low value of the dielectric constant. But the intensity of the 1740 cm-1 band is low as compared to the similar band in the case of associated aminoporphyrin as a charge transfer complex is formed in the corresponding thin films [4]. This band is absent in the previous spectra since other organic solvents (acetone and tetrahydrofuran) are almost completely evaporated from the corresponding thin films. Conclusions - the decrease of the 437 nm band accompanied by the aggregation of protonated TPP dimers, is due to the aggregation process or/and a photoreaction between different dimers in the porphyrin aggregates observed earlier; - the separate aggregates with the sizes of one-several micrometers formed in different water-organic solutions, has usually quite flat surface although a rough surface is sometimes seen on the aggregates; - the doublet of deformational vibrations of water (strong 1610 cm-1 and weak 1632 cm-1 bands) suggests all water molecules are bound in the porphyrin aggregates and involved in donor-acceptor interactions with porphyrin molecules and other water molecules forming a continuos microphase; - the presence of the doublet of deformational vibrations is accompanied by the doublet of valence vibrations of water with the strong 3512 cm-1 and weak 3457 cm-1 bands, so that the bands of valence vibrations have the mirror reflection of the corresponding deformational vibrations; - a new broad band with the maximum located within 2136-2152 cm-1 region, but not seen in the spectrum of TAPP associated in thin film, is revealed in the IR spectra of protonated TPP aggregates; - one and the same frequency (3220 cm-1) of the valence nN-H vibrations of the bonds of pyrrole ring observed in the both porphyrins, suggests similar character of interaction between water and macrocycle of the metal-free porphyrins, when the frequency is red shifted by 95 cm-1 as compared to neutral TPP (for the latter the frequency of nN-H vibrations equals 3315 cm-1). References