Trapping of the radical products from the organic azide photooxidation reaction mixture
Maxim V. Kuznetsov, Sergei V. Zelentsov,
Alexander A. Shchepalov
Nizhnii Novgorod State University, Chem. Department,
Gagarin Ave., 23. Nizhnii Novgorod 603600 Russia
E-mail: zelen@ichem.unn.runnet.ru
Summary. Radical intermediates from 4,4’-diazidobiphenyl photooxidation in the benzene solutions saturated with molecular oxygen were trapped with 2-methyl-2-nitrosopropane. Simulation of the ESR spectra observed suggests that they correspond to an adduct of phenyl radical and spin trap. The spectrum can be assigned to the system consisting of 2 pairs equivalent protons having super fine couplings of 2.43 and 1.16 G and of one nitrogen with aN = 14.9 G.
Keywords: ESR spectra, spin trap, photoxidation, aromatic azide, radical intermediate.
1. Introduction.
One of the main problems of the organic azides photooxidation study is to determine the chemical nature of the intermediates taking part in the reaction, particularly it is intriguing to investigate the adduct of a nitrene and molecular oxygen [1,2]. The adducts can be stabilized into the final products with involvement of both the triplet, RNOOT, and singlet, RNOOS, states.
Triplet route existence has been proved in triplet photosensitization experiments [2,3], but the RNOOT secondary reactions are not studied yet. And the main question remaining uncleared is whether the molecules of the reaction media are able to be the source of hydrogen atoms and thus generate radicals.
The aim of the present paper was to determine the chemical nature of the radical intermediates involved in the organic azide photooxidation which were trapped as adducts with spin traps such as 2-methyl-2-nitrosopropane (1).
2. Experimental part.
The photooxidation of 4,4’-diazidodiphenyl in benzene solution containing CH2Cl2 was performed in the quartz photochemical reactor through which dried air was bubbled. 2-methyl-2-nitrosopropane was used as a spin trap. (The photooxidation runs were perform by A.B.Zhezlov, M.S. They will be described in details elsewhere. The authors thank Mr. Zhezlov for his technical assistance).
The irradiated solution was placed into ESR registration tube. Measurements of ESR spectra were done using AE 4700 radiospectrometer of 3 cm range. Parameters of the registration were modulation amplitude of 200 mG, MW intensity attenuating of the 4 dB, temperature of 298 K.
WINSIM program [4] was used to simulate ESR spectra. Simplex method was used to determine the best ESR parameter set, the parameters from [5] being used as the first approximation. The shape of an individual line was chosen in such a way which gave a ratio of contributions of the Gauss and Lorentz lines being of 0.9:0.1, as it was well known that the latter ratio was optimal for spin radicals in liquids having low viscosity [6].
Electronic structures of the structures proposed were calculated by means of quantum chemistry method. UHF/6-31G(d) method using the Meller-Placet second order perturbation theory (MP2) to account for electron correlation was used. Some calculations used B3LYP/6-31D(d) method. Gaussian 94 program [7] installed in Super Computer Center in IOC organization of RAS was used.
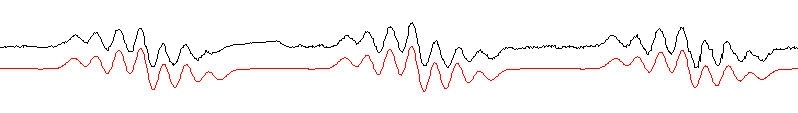
Simulation procedure revealed that the most optimal ( correlation coefficient between simulated and experimental spectrum is 0.95) is model that consisting of two pairs of equivalent protons having hyper fine splitting of 2.43 G and 1.16 G and nitrogen splitting of 14.9 G. The simulated and experimental spectra are shown on Figure 1.

Figure1. The experimental (black line) and simulated (red line) spectrum.
The two structure corresponding to the parameters found are shown on Fig. 2. The structure can be:
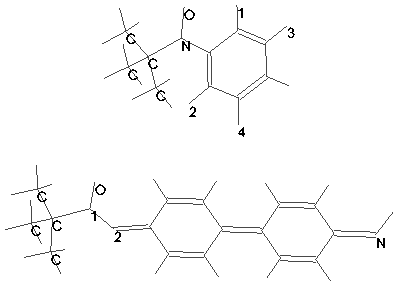
Figure 2. The nitrene-oxygen adducts structures. The top figure shows the adduct of the spin trap and phenyl radical. The structure shown below corresponds the adduct of spin trap and 4,4’-dinitrene diphenyl.
The latter becomes possible only in the case when there is a node of wave function located on the said atom.
The quantum chemical calculations of the Fermi contact spin densities ( Table 1 ) gave us an opportunity to eliminate from our consideration the second structure because of spin density on the nitrogen atom directly connected with nitroxyl nitrogen is not zero ( See line 2 for structure 2 in Table 1).
Table 1.
Fermi contact spin densities for structure 1 and structure 2.
structure 1 | structure 2 | ||
atom | spin density | atom | spin density |
H1 | -0.0019 | N1 | 0.0322 |
H2 | -0.0018 | N2 | -0.0262 |
H3 | 0.0007 | ||
H4 | 0.0007 | ||
Spin densities on proton 1 and 2 being in ortho-position of the first structure are the same. This can prove their equivalence. The same can be said about protons 3 and 4 being in metha-position of the structure. Spin density on metha-protons have the absolute value of one decimal power lower than the value for ortho-protons. This suggests unambiguosly that the hyper fine splitting of 2.43 G caused by splitting on the ortho-protons, and the one of 1.16 G caused by splitting on the metha-protons.
Our results give unambiguous evidence of phenyl radical participation in the organic azide photooxidation. It can emerge from an hydrogen abstraction reaction of PhNOOT with hydrogen containing molecules of the reaction mixture:
RNOOT + C6H6 ® R•NOOH + C6H5• (1)
It was namely Ph• radical particles that were trapped in our spin trapping experiments. Further step should be N-radical hydroperoxide decomposition to produce nitrosocompound and hydroxyl radical [1,8].
R•NOOH ® RNO + •OH (2)
Hydroxyl can recombine with phenyl radical to produce phenol. Both nitrosocompound and phenol were found among final product of the organic azide photooxidation at low conversion [9,10].
C6H5• + •OH ® C6H5OH (3)
It seems to be the first experimental evidence of participation of the radical products emerged from an hydrogen atom abstraction reaction. We shall continue our experiments to trap other radical that could possibly be involved in the reaction.
Acknowledgment. The work was done under financial support Scientific Program "Russian University - Basic Researches" (project 015.05.01.38).
References.
1. S.V.Zelentsov, N.V. Zelentsova, A.B.Zhezlov, and A.V.Oleinik//High energy chemistry, 34(2000),201.
2. S.V.Zelentsov, A.B.Zhezlov. The 18th IUPAC Symp. on Photochemistry. Dresden, July 22-27, 2000. Book of abstracts. Dresden Technische Universitat. 2000. P.653.
3. S.V.Zelentsov. International Conf. “Organometallic Compounds – The materials of the Future Millennium” (The third Razuvaev Lectures). Nizhnii Novgorod, 29 May – 2 June, 2000. Book of Abstracts. Nizhnii Novgorod. 2000.P.165.
4. D.R.Duling// J. Mag. Res. B, 104 (1994), 105.
5. V.E.Zubarev The spin trap method. Moscow. MSU Publ. 1984 (in Russ.).P.188.
6. J.H.Freed // Spin labeling: Theory and Application / Ed.L.Berliner. Academic Press. New York,1976.
7. J. Frisch, G. W. Trucks, H. B. Schlegel, P. M. W. Gill, B. G. Johnson, M. A. Robb, J. R. Cheeseman, T. A. Keith, G. A. Petersson, J. A. Montgomery, K. Raghavachari, M. A. Al-Laham, V. G. Zakrzewski, J. V. Ortiz, J. B. Foresman, J. Cioslowski, B. B. Stefanov, A. Nanayakkara, M. Challacombe, C. Y. Peng, P. Y. Ayala, W. Chen, M. W. Wong, J. L. Andres, E. S. Replogle, R. Gomperts, R. L. Martin, D. J. Fox, J. S. Binkley, D. J. Defrees, J. Baker, J. P. Stewart, M. Head-Gordon, C. Gonzalez and J. A. Pople, Gaussian 94 (Gaussian, Inc., Pittsburgh, PA, 1995).
8. S.V.Zelentsov//The 6th All Union Symp. on Photochemistry. Novosibirsk. 16-18 May 1989. Novosibirsk. ICKC SD AS USSR. 1989. Book of Abstracts. Part 1. P.168. (in Russ.).
9. Yasuhiro Suwaki, Shinji Ishikawa, and Hiizu Iwamura //J.Am.Chem.Soc. 109(1987), 584.
10. C.Lee Go and W.H.Waddell // J.Org. Chem. 48(1983), 2897.