| Papers and Posters | Site Home Page |
Protection against UV-induced systemic immunosuppression
by commercially-available sunscreens.
Authors: J.J. Finlay-Jones*, A. Jaksic, L.K. Spencer, I. Santucci, and P.H. Hart
Department of Microbiology and Infectious Diseases, School of Medicine, Faculty of Health Sciences, Flinders University of South Australia, G.P.O. Box 2100, Adelaide, South Australia, 5001.
* Tel + 61 8 8204 4292; Fax + 61 8 8276 8658; Email John.Finlay-Jones@flinders.edu.au
Summary
Sunscreens have varying abilities to protect against the immunomodulation that can follow ultraviolet irradiation of skin. This immunomodulation, which can be demonstrated in murine model systems as suppression of contact hypersensitivity (CHS) responses, may be defined as local or systemic. This study examined the properties of several commercially-available, SPF 15+, broad-spectrum sunscreens, which were applied to the shaved backs of BALB/c mice prior to UV irradiation (5-18 kJ/m2). All sunscreen applications protected against the acute effects of UV exposure, but varied in the protection offered against systemic immunosuppression.Introduction
The adverse effects of UVB radiation (UVR) of the skin include [1,2]:
| erythema, | |
| photoageing, and | |
| induction of skin cancer. |
In experimental models, the outgrowth of UV-induced skin cancer is associated with immunomodulation (or "immunosuppression") induced by UVR [3]. This immunomodulation allows the progressive growth of what are typically immunogenic tumors, which would otherwise be rejected by an anti-tumor immune response. In humans as in animal models, UVR is immunomodulatory [4,5]. In addition, humans with skin cancer are more susceptible to the immunosuppressive effects of UVR [6].
The immunosuppressive effects of UVR are either:
| local (antigen delivery at the irradiated site) or | |
| systemic (antigen challenge at an unirradiated site). |
The mechanism of local immunosuppression following UVR involves effects of UVR on Langerhans cells [7], whereas the sequence of events for systemic immunosuppression involves cis-urocanic acid (cis-UCA [8]) and dermal mast cells [9]. Whilst with local immunosuppression the diminished response to antigen can be shown by sensitizing via the affected site immediately following irradiation, systemic immunosuppression does not develop until 1 to 3 days after irradiation [10].
Broad spectrum, high SPF (sun protection factor – e.g., 15+, 30+) sunscreens provide protection against many of the acute and chronic effects of UVR, including erythema and photoageing [1]. However, reports vary with respect to the ability of sunscreens to protect against immunomodulation (see review [11]).
Typical studies have been conducted in mice using models of contact hypersensitivity (CHS), both local [12,13,14,15,16,17] and systemic [14, 17, 18, 19,20], as well as a model of delayed-type hypersensitivity (DTH [21]). Protection has varied from minimal (see, e.g., [12, 19]) to total [17] using a range of sunscreen preparations, both commercially available and experimental. Sunscreen properties correlating with protection are not clear.
This study presents our findings using several commercially-available sunscreens.
Materials and Methods
Details of most methods are found elsewhere [9]. In brief:
Mice. Female BALB/c mice 8-12 weeks of age.
UVR Irradiation. Westinghouse FS40 sunlamps were used, located 20 cm above the irradiation area, with a sheet of polyvinyl chloride (PVC) plastic (0.4 mm) as a filter to remove UVR of wavelengths < 293 nm (determined by spectral radiometry; various palstic films were screened for this property, and for its stability during the period of irradiation). For routine experimentation, the dose was determined using a UVX radiometer and UVX 310 detector (Ultraviolet Products Inc., San Gabriel, CA), and was delivered at a rate of 0.5 W.m-2 onto an area of shaved skin of approximately 8 cm2.
Reagents. The trans isomer of UCA was purchased from Sigma (St Louis, MO). Cis-UCA was purified from irradiated trans-UCA by ion-exchange chromatography.
Sunscreen preparations. A range of commercially-available sunscreen preparations was obtained. There were two SPF 15+ products, an SPF 30+ product and an SPF45 product. The products denoted SPF15+ and 30+ were obtained in Australia and their SPF designations reflect labeling conventions in that country. The SPF 45 product was obtained in the USA.
Sunscreen A: a broad spectrum, alcohol-based sunscreen containing 2-ethyl hexyl-paramethoxy cinnamate, 2-hydroxy-4-methoxy-benzophenone and 4-tert-butyl-4-methoxy dibenzoyl methane, which had a stated SPF of 15+, and was approximately 18.
Sunscreen B: a broad spectrum, SPF 15+sunscreen in a milky lotion base having the same active ingredients as sunscreen A
Sunscreen C: a broad spectrum, SPF 30+ sunscreen containing octyl methoxycinnamate, 4-methylbenzylidene camphor, butyl methoxydibenzoylmethane and titanium dioxide.
Sunscreen D: a broad spectrum, SPF45 sunscreen containing ethylhexyl p-methoxycinnamate, octocrylene, oxybenzone and 2-ethylhexyl salicylate.
Alcohol- and milky-base lotions were obtained from a manufacturer and used as control treatments.
Contact Hypersensitivity (CHS) Responses and Their Systemic Suppression by UV Irradiation. Mice (n=5 per group) were sensitised on the shaved ventral skin with 100 m l freshly-prepared 5% (w/v) 2,4,6-trinitrochlorobenzene (TNCB, Tokyo Kasei Kogyo Co. Ltd., Tokyo, Japan) in acetone. Five days later, a CHS response was elicited by applying 10 m l freshly prepared 1% TNCB in acetone to each of the ventral and dorsal surfaces of both ears. Twenty-four hours after challenge, the ear thickness was measured with a micrometer, and the extent of ear swelling for each mouse was calculated by subtracting the ear thickness measured before challenge. In experiments measuring systemic immunosuppression by UVR, mice (with prior application of 50 m l sunscreen or base lotion, as indicated, and with ears protected with black electrical insulation tape) were UV-irradiated on shaved dorsal skin, and 5 days later a CHS assay was performed.
Determination of UCA content of skin by HPLC. Punch biopsies of skin were alkali-extracted, then acid treated and evaluated by HPLC [22] against known standards.
Statistics. Results are presented as mean values ± SD. Data were analysed by one way analysis of variance. Significant differences between means were subsequently determined by multiple classification analysis or by Student's t-test.
Results
All sunscreen preparations used protected against the erythema/edema response of murine skin following UV exposure at the doses used.
UV-induced suppression of systemic CHS responses.
Sunscreen A was evaluated for its ability to protect against UV-induced immunosuppression.
UVR at doses of 10 or 15 kJ/m2 induced significant systemic immunosuppression in both base- and sunscreen-A-pretreated mice, with the sunscreen providing only partial protection against immunosuppression, more evident at the lower dose (Table 1).
Table 1: Contact hypersensitivity Responses in UV-Irradiated Mice: Sunscreen A
UV Dose (kJ/m2) |
Base |
Sunscreen A |
0 |
0.24 ± 0.04 (-) 1 |
0.28 ± 0.05 (-) |
5 |
0.25 ± 0.04 (-7%) |
0.25 ± 0.08 (12%) |
10 |
0.14 ± 0.04 (41%) 2 |
0.22 ± 0.05 (21%) 2 |
15 |
0.12 ± 0.03 (51%) 2 |
0.17 ± 0.02 (42%) 2,3 |
1
Data are presented as ear swelling in mm, mean ± sd, with % suppression in parentheses (calculated as [100 - % of relevant control response]).2
Significantly less than relevant control (P < 0.05)3
Significantly less than response at 10 kJ/m2Immunoprotection by Sunscreens and Formation of cis-UCA.
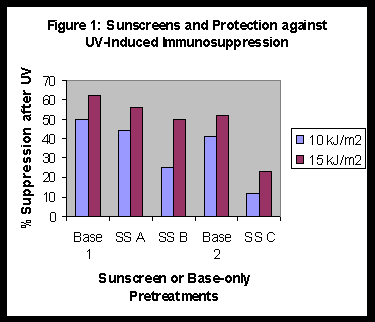
In a series of experiments we evaluated Sunscreens A, B and C for protection against systemic immunosuppression. The results are summarised in Figure 1. Sunscreen C was more protective than the other two.
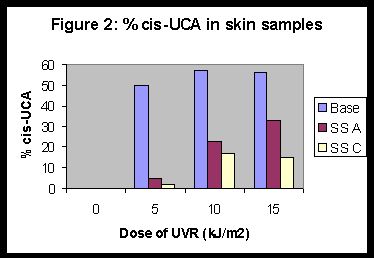
In a separate experiment, cis-UCA levels in skin immediately following irradiation were quantified in mice given prior application of either a base lotion or a sunscreen A or C. Cis-UCA levels in base-lotion-treated, irradiated skin were significantly higher than in sunscreen-pretreated skin at all doses of UVR examined (Figure 2).
In general it has not been possible to correlate immunoprotection with SPF [11]. Nevertheless, to explore this, we compared sunscreen C and sunscreen D. There was a significant difference in the immunoprotection afforded by the two formulations (Table 2), even though the difference in the amount of erythemally active UVR transmitted by the two would be less than 2% of the incident irradiation.
Table 2: Effect of High-SPF Sunscreens on UV-induced Systemic Immunosuppression*
UVR (12 kJ/m2) |
Pre-UVR Treatment |
Ear Swelling (mm) (mean ± sd) |
% suppression 1 |
- |
None |
0.24 ± 0.03 |
0 |
+ |
None |
0.16 ± 0.02 2 |
33 |
+ |
SPF 30+ |
0.22 ± 0.04 3 |
8 |
+ |
SPF 45 |
0.18 ± 0.05 2,4 |
25 |
1
calculated as (100 - % of control response)2
significantly different to unirradiated control, Fisher’s least significant difference test (p<0.05)3
not significantly different to unirradiated control4
not significantly different to irradiated control
Discussion
Using a model of UV-induced suppression of a systemic CHS response in BALB/c mice, we have evaluated the immunoprotective properties of several commercially-available, broad spectrum, high SPF sunscreens.
The key finding was that the sunscreens varied markedly in their abilities to protect against immunosuppression.
Strains of mice differ in their susceptibility to UVR, based on the dose of UV radiation required for 50% suppression of the systemic contact hypersensitivity response [23].
C57BL/6 and C57BL/10 mice are examples of high susceptibility strains (requiring 1.7-2.3 kJ/m2 for 50% suppression), DBA/2, C3H/HeJ and C3H/HeN strains are intermediate in susceptibility (requiring 4.7-6.0 kJ/m2), and the strain used, BALB/c, is of low susceptibility (9.3 kJ/m2 for males, 12.6 kJ/m2 for females).
How much UVR would be transmitted by these high SPF sunscreens, and would this be enough to induce immunosuppression in the strain used, BALB/c? This is not easily answered, as a detailed consideration would require analysis of the absorption spectrum of each sunscreen preparation, the action spectrum of erythema and of systemic immunosuppression, and the incident radiation.
As an approximation, and referring to at least the erythemal radiation, no more than 1 kJ/m2 of a 15 kJ/m2 exposure should be transmitted through an SPF 15 sunscreen. Regression analysis of "% suppression" vs "log UV dose" [23] indicates that 1 kJ/m2 would cause no detectable immunosuppression in BALB/c mice (here we’re assuming the filtration of the erythemally active radiation reflects that of immunomodulatory wavelengths); further, a dose of 5 kJ/m2 did not produce significant systemic suppression (Table 1). Therefore, the immunosuppression seen in sunscreen-treated mice appears not to be explained by sufficient UVR being transmitted directly through the sunscreen layer. Marked differences in sunscreen performance in filtering out the more immunosuppressive wavelengths at the same time as much less marked differences with respect to erythemal wavelengths might explain the outcome, especially if one were dealing with simpler, experimental sunscreen prreparations. However, the most effective wavelengths for systemic immunosuppression [8] are below 290 nm (not present in the radiation applied), and the sunscreens tested had combinations of active ingredients to augment filtration. Also, the possibilities whereby sunscreen formulations may vary in immunoprotection clearly relate to underlying mechanisms of immunosuppression, discussed below.
The trans isomer of urocanic acid (deaminated histidine) is a naturally-occurring substance found in the stratum corneum, and is one of several targets for UV irradiation of skin from which immunomodulatory responses can be generated [24]. UV irradiation of the skin results in the formation of the cis isomer (the dominant isomer at steady state), and this latter molecule is itself immunomodulatory, in contrast to the trans isomer [24].
Although there was evidence that sunscreens A and C could diminish the % of cis-UCA formed after UV irradiation, the differences were not sufficient to explain the differences in immunoprotection offered. This should be taken together with the evidence that the formation of cis-UCA in skin was not in itself sufficient to induce immunosuppression. A dose of 5 kJ/m2 of UV irradiation was sufficient to induce isomerisation of urocanic acid to >50% cis in irradiated skin (Figure 2), but this was insufficient to induce immunosuppression (Table 1). In other models it has been possible to dissociate the formation of cis-UCA in the skin from immunosuppression, for example with UVA-irradiation [25].
That an ability to abrogate immunosuppression does not relate simply to, e.g., the SPF value of a sunscreen was demonstrated by the finding that an SPF 45 sunscreen did not abrogate immunosuppression in the model used (Table 2). What accounts for the difference in immunoprotection offered by sunscreens which are otherwise highly protective against erythema/edema is unknown. Following an analysis of many published studies, Young and Walker [11] concluded that such differences could not be ascribed to a specific sunscreen molecule. Given the properties of Sunscreen C (marketed as Hamilton ‘Superblock’), we worked with the manufacturer (Hamilton Laboratories, South Australia), in attempts to define the protective component(s). Studies of individual active chemicals and combinations in defined vehicles did not reveal the key chemical or combination, leading to the conclusion that the formulation of the product as well as its active chemical components was important.
Conclusions
We have recently shown that the induction of systemic (but not local) immunosuppression by UVR is critically dependent on the presence of dermal mast cells [9], and we have hypothesised that cis-UCA may trigger mast cell degranulation to initiate immunosuppression [26], a pathway that depends at least in part on histamine [27] and prostanoid induction [28].
We would hypothesise, then, that the ability of a sunscreen formulation to protect against induction of systemic immunosuppression by UVR might depend, inter alia, on inhibition of isomerisation of trans- to cis-UCA. Failing that, prevention of the degranulation of dermal mast cells by the induced cis-UCA, by whatever mechanism, or inhibition of subsequent pathways, would also lead to protection. These latter possibilities might also explain the ability of UVA irradiation to induce trans- to cis-UCA isomerisation yet not induce immunosuppression [25].
Acknowledgements
Our work has been supported by grants from the Anti-Cancer Foundation of South Australia, the National Health and Medical Research Council of Australia, and the Flinders Medical Centre Foundation. In addition, through Flinders Technologies Pty Ltd, we have collaborated with Hamilton Laboratories Pty Ltd in studies of sunscreen evaluation and development, supported in part by grants (National Teaching Company Scheme, Australian Postgraduate Research Award with Industry, and Generic Industrial Research and Development) from several Australian Government Departments.
References
[1] Taylor, C.R., R.S. Stern, J.J. Leyden, and B.A. Gilchrest (1990) Photoaging/ photodamage and photoprotection. J. Amer. Acad. Dermatol. 22, 1-15.
[2] Taylor, C.R. and A.J. Sober (1996) Sun exposure and skin disease. Annu. Rev. Med. 47, 181-191.
[3] Kripke, M.L. (1984) Immunological unresponsiveness induced by ultraviolet radiation. Immunological Reviews 80, 87-102.
[4] Hersey, P., G. Haran, E. Hasic and A. Edwards (1983) Alteration of T cell subsets and induction of suppressor T cell activity in normal subjects after exposure to sunlight. J. Immunol. 131, 171-174.
[5] Baadsgaard, O. (1991) In vivo ultraviolet irradiation of human skin results in profound perturbation of the immune system. Relevance to ultraviolet-induced skin cancer. Arch. Dermatol. 127, 99-109.
[6] Streilein, J.W., J.R. Taylor, V. Vincek, I. Kurimoto, J. Richardson, C. Tie, J.-P. Medema, C. Golomb (1994) Relationship between ultraviolet radiation-induced immunosuppression and carcinogenesis. J. Invest. Dermatol., 103, 107S-111S.
[7] Streilein, J.W., J.R. Taylor, V. Vincek, I. Kurimoto, T. Shimizu, C. Tie and C. Golomb (1994) Immune surveillance and sunlight-induced skin cancer. Immunology Today 15, 174-179.
[8] De Fabo, E.C., F.P. Noonan (1983) Mechanism of immune suppression by ultraviolet irradiation in vivo. 1. Evidence for the existence of a unique photoreceptor in skin and its role in photoimmunology. J Exp. Med., 158, 84-98.
[9] Hart, P.H., M.A. Grimbaldeston, G.J. Swift, A. Jaksic, F.P. Noonan and J.J. Finlay-Jones (1998) Dermal mast cells determine susceptibility to ultraviolet B-induced systemic suppression of contact hypersensitivity responses in mice. J. Exp. Med. 187, 2045-2053.
[10] Noonan, F.P., M.L. Kripke, G.M. Pedersen and M.I. Greene (1981) Suppression of contact hypersensitivity in mice by ultraviolet irradiation is associated with defective antigen presentation. Immunology 43, 527-533.
[11] Young, A.R. and S.L. Walker (1995) Photoprotection from UVR-induced immunosuppression. In: Photoimmunology (Edited by J. Krutmann and C.A. Elmets), pp 285-297. Blackwell Science, Oxford.
[12] Lynch, D.H., M.F. Gurish and R.A. Daynes (1981) Relationship between epidermal Langerhans cell density ATPase activity and the induction of contact hypersensitivity. J. Immunol. 126, 1892-1897.
[13] Ho, K.K-L., G.M. Halliday, R. St.C. Barnetson (1992) Sunscreens protect epidermal Langerhans cells and Thy-1+ cells but not local contact sensitization from the effects of ultraviolet light. J. Invest. Dermatol. 98, 720-724.
[14] Wolf, P., C.K. Donawho and M.L. Kripke (1993) Analysis of the protective effect of different sunscreens on ultraviolet radiation-induced local and systemic suppression of contact hypersensitivity and inflammatory responses in mice. J. Invest. Dermatol. 100, 254-259.
[15] Bestak, R., R. St.C. Barnetson, M.R. Nearn and G.M. Halliday (1995) Sunscreen protection of contact hypersensitivity responses from chronic solar-simulated ultraviolet irradiation correlates with the absorption spectrum of the sunscreen. J. Invest. Dermatol. 105, 345-351.
[16] Roberts, L.K. and D.G. Beasley (1995) Commercial sunscreen lotions prevent ultraviolet-radiation-induced immune suppression of contact hypersensitivity. J. Invest. Dermatol. 105, 339-344.
[17] Roberts, L.K., D.G. Beasley, D.B. Learn, L.D. Giddens, J. Beard and J.W. Stanfield (1996) Ultraviolet spectral energy differences affect the ability of sunscreen lotions to prevent ultraviolet-radiation-induced immunosuppression. Photochem. Photobiol. 63, 874-884.
[18] Morison, W.L. (1984) The effect of a sunscreen containing para-aminobenzoic acid on the systemic immunologic alterations induced in mice by exposure to UVB radiation. J. Invest. Dermatol. 83, 405-408.
[19] Fisher, M.S., J.M. Menter and I. Willis (1989) Ultraviolet radiation-induced suppression of contact hypersensitivity in relation to padimate O and oxybenzone. J. Invest. Dermatol. 92, 337-341.
[20] Reeve, V.E., M. Bosnic, C. Boehm-Wilcox and R.D. Ley (1991) Differential protection by two sunscreens from UV radiation-induced immunosuppression. J. Invest. Dermatol. 97, 624-628.
[21] Wolf, P., D.B. Yarosh and M.L. Kripke (1993) Effects of sunscreens and a DNA excision repair enzyme on ultraviolet radiation-induced inflammation, immune suppression, and cyclobutane pyrimidine dimer formation in mice. J. Invest. Dermatol. 101, 523-527.
[22] Morrison, H., D. Avnir and T. Zarrella (1980) Analysis of Z and E isomers of urocanic acid by high-performance liquid chromatography. J. Chromatogr. 183, 83-86.
[23] Noonan, F.P. and H.A. Hoffman (1994) Susceptibility to immunosuppression by ultraviolet B radiation in the mouse. Immunogenetics 39, 29-39.
[24] Noonan, F.P. and E.C. De Fabo (1992) Immunosuppression by ultraviolet B radiation: initiation by urocanic acid. Immunology Today 13, 250-254.
[25] Webber, L.J., E. Whang and E.C. De Fabo (1997) The effects of UVA-I (340-400 nm), UVA-II (320-340 nm) and UVA-I+II on the photoisomerization of urocanic acid in vivo. Photochem. Photobiol. 66, 484-492.
[26] Finlay-Jones, J.J. and P.H. Hart (1997) Ultraviolet irradiation, systemic immunosuppression and skin cancer: role of urocanic acid. Australas. J. Dermatol. 38, S7-S12.
[27] Hart, P.H., A. Jaksic, G. Swift, M. Norval, A.A. El-Ghorr and J.J. Finlay-Jones (1997) Histamine involvement in UVB- and cis-urocanic acid-induced systemic suppression of contact hypersensitivity responses. Immunology 91, 601-608.
[28] Jaksic, A., J.J. Finlay-Jones, C.J. Watson, L.K. Spencer, I. Santucci and P.H. Hart (1995) Cis-urocanic acid synergizes with histamine for increased PGE2 production by human keratinocytes: link to indomethacin-inhibitable UVB-induced immunosuppression. Photochem. Photobiol. 61, 303-309.