| Photoiupac home page | Discussion | Photobiology.com home |
FLUORESCENCE EMISSION STUDIES ON PHOTODEGRADATION OF PHENOL UNDER DIRECT AND CONCENTRATED SUNLIGHT
Bircan Dindar and Sıddık Içli
Department of Chemistry, Faculty of Science, Ege University,35100 Bornova, Izmir Turkey
INTRODUCTION :
Phenol and phenolic compounds are common contaminants in industrial wastewaters. Phenolic contaminants are generated from the processes washings of coke ovens and most engineering workshops. In aqueous solutions, we have studied the degradation of phenol in presence of TiO2, Fe2O3 and ZnO . Irradiations have been done in photoreactors under sodium lamp, direct sunlight, and concentrated sunlight.
In the presence of adsorbed oxygen and an electron donor, chemical reactions can occur on the surface of the metal oxide particles ( M Ox ), such as TiO2, Fe2O3 and ZnO .
hn
M Ox M Ox ( ecb - + hvb + ) ( 1 )
< 380 nm
M Ox ( e- ) + O2 (s) O2 .-(s) ( 2 )
An electron transfer has been carried out from adsorbed solvent molecules ( H2O and OH- ).
M Ox ( h+ ) + OH- (s) .OH (s) + M Ox ( 3 )
The generated radical can attack to phenol molecules to produce o- and p- dihydroxy phenols.
.
OH(s) + M Ox ( h+ ) + C6 H5 OH (s)
HO
.C6 H5 OH + M Ox ( e- ) + H+ ( 4 )
Organic pollutants adsorbed onto the surface of the metal oxide particles will be oxidised by .OH radicals. Further, it has given rise to formation of other oxidation compounds and subsequent opening of the aromatic ring and continued with formation of carbon dioxide.
HO . C6 H5 OH(s) + O2
HO C6 H5 OH . O2 (s) ( 5 )
C6 H5 OH (s) + 7O2 6CO2 + 3H2O ( 6 )
Solar photodegradaton of phenol by metal oxides (TiO2, Fe2O3 and ZnO ) under concentrated sunlight is not reported in literature. We now presents the results of photodegradation of phenol rates under direct and concentrated sunlight.
MATERIALS AND METHODS :
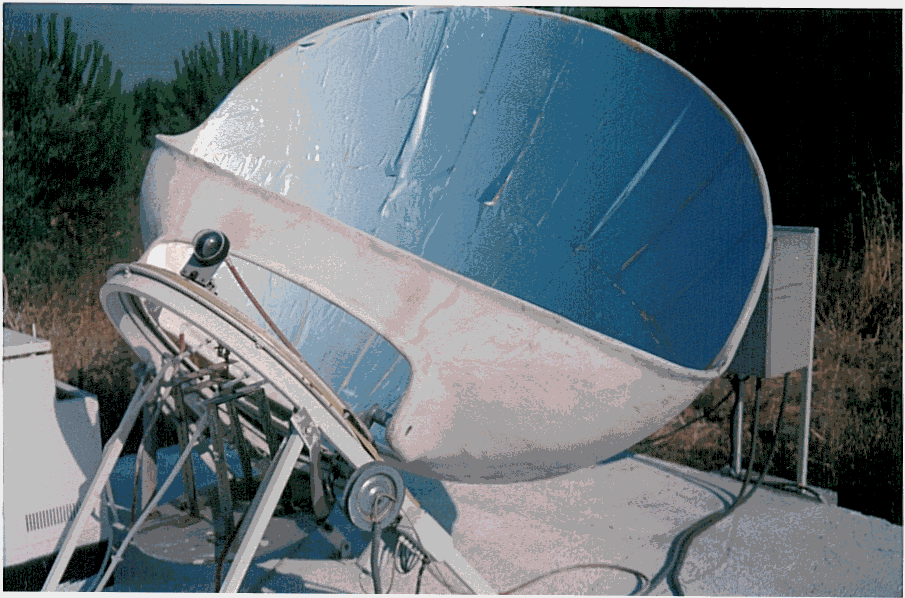
A cylindrical pyrex glass reactor with tap on the bottom was used as a photoreactor. The experimental photoreactor system equipped with a 400 W Sodium Lamp is shown in Figure 1. PHILIPS- SON–T PLUS- 400 W Sodium Lamp ( Belgium ) was used for visible radiation source and FIX FOCUS FF 3.5 ( HTC GmbH, Germany ) sun collector system was used for the concentration of sunlight. The technical specifications; the area of total reflective surface is 3.68 m2, useable reflector area is 2.66 m2 , focal length is 0.65 m, 3M SA85P a reflective film aluminised with a percent of initial reflectivity; 85 %. The solar concentrator is seen in Picture 1.

Picture 1. Automatically focused solar concentrator.
Irradiation intensities of light sources were measured with a Vilbert Lourmat VLX 312 nm Radiometer and were about 0.2 sun in solar photoreactor, 1 sun under direct sunlight, and 40 - 50 suns in solar concentrator, during april-september period between 11.00 and 14.00 hours.
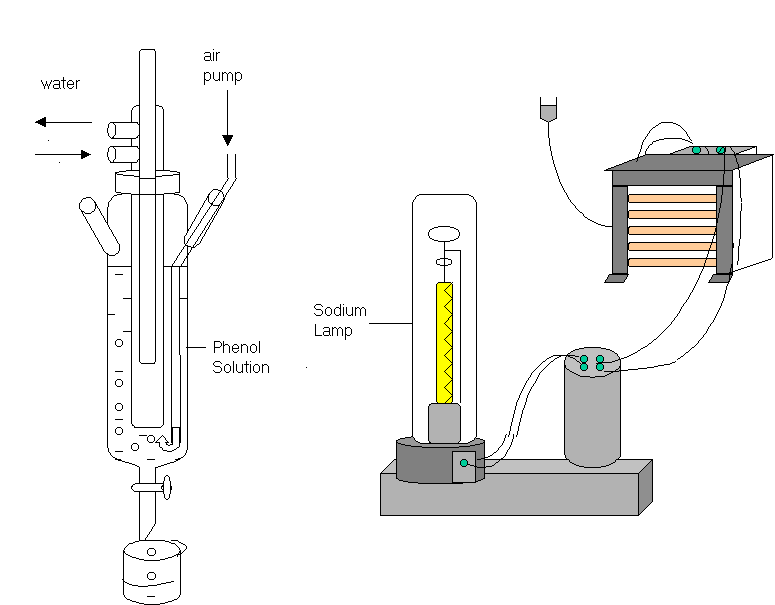
Figure 1 . A micro solar simulation system equipped with a 400 W sodium lamp.
125 ml of phenol solution was illuminated in the photoreactor system . Sodium lamp was placed in 4.0 cm distance from the side wall of the pyrex glass reactor. The inlet pump tubing was located on the bottom of the solution. Whole
system was placed in a cubic room which has reflective surfaces. The cubic room
have diameters of 50 cm x 50 cm x 50 cm.
Aeration was provided by the use of an aquarium pump during experiments. The air pumping, not only prevents the precipitation of semiconductor particles, but also provides oxygen into the solution continously. Solutions were cooled
with running water at outer chambers during illumination and thus, temperature increase
of solutions were kept at minimal levels.
Before illumination, samples with and without photocatalyzer were measured for phenol content, at absorbtion and
fluorescence emission spectra. A volume of 5 ml samples were taken out during irradiations at 5, 10, 15, 20, 25, 30, 45, 60 and 90 minutes intervals for spectroscopic measurements either in photoreactor or under direct sun light. In
Solar Concentrator, the samples were taken out at every 3 minutes in 15 minutes
period. Samples taken out for spectroscopic measurements were not reused.
The samples from solutions were always filtrated with Blue Ribbon filter paper,
prior to spectroscopic measurements. Reference measurements from non - radiated samples were compared with samples obtained from direct sun light, sodium lamp photoreactors and solar concentrator system.
Phenol degradation have been determined both by decrease of phenol fluorescence emission (l = 296 nm) and diminution of UV absorption(l =460 nm) at 4-aminoantipyrine method.
RESULTS AND DISCUSSIONS :
Photodegradation of phenol has been studied in presence of metal oxides of TiO2, Fe2O3 and ZnO and under direct and concentrated sunlight and in a solar photoreactor system.
In the fluorescence spectrums of samples obtained by using Solar Concentrator System, disappearance of phenol also has been seen shorter time than other systems as following in respectively; ( 1 ) Standard phenol solution, ( 2 ) No irradiation in the presence of photosensitiser, ( 3 ) 3 min. irradiation, ( 4 ) 6 min. irradiation ( 5 ) 9 min. irradiation, ( 6 ) 12 min. irradiation, ( 7 ) 15 min. Fluorescence spectra on degradation of phenol with ZnO are shown in Figure 2 below.
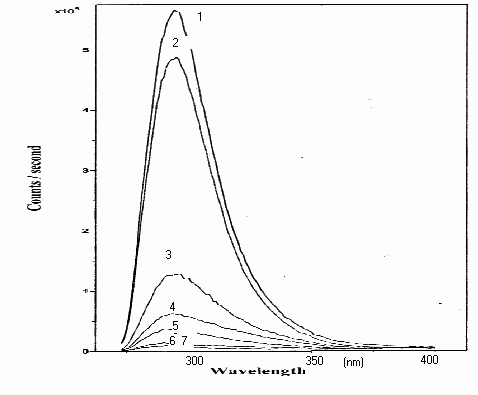
Figure 2. Fluorescence spectra on degradation of phenol at 5. 10-5 M concentration with 0.5 g l-1 of ZnO photocatalyzer, under concentrated sunlight ( in 15 minutes period).
The diminution of the concentration of phenol was determinated as a function of irradiation. Overall results are shown in Table 1 and 2 below.
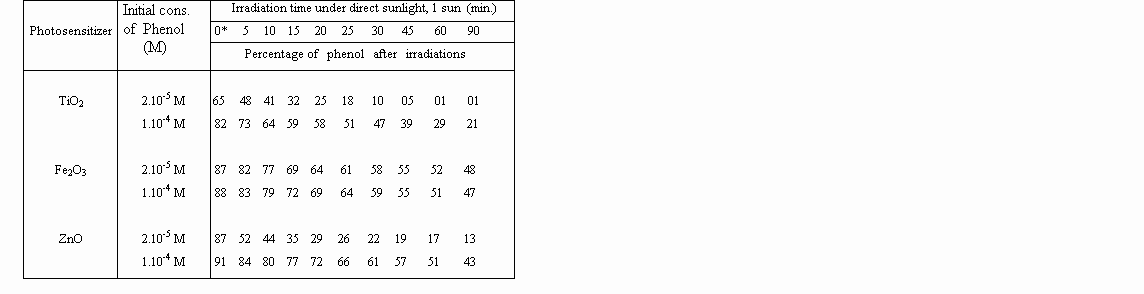
Table 1: Percentage of degraded phenol during the course of direct solar irradiation
(1 sun) by the use of metal oxide photosensitizers, in aqueous solution.
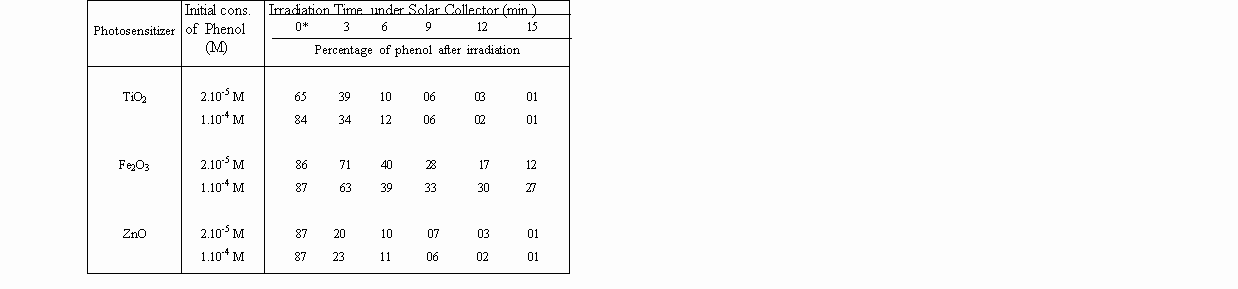
Table 2. Percentage of degraded phenol in Solar Concentrator System (40-50 suns)
by the use metal oxide photosensitizers, in aqueous solution.
A striking observation is that, zinc oxide is as reactive as titanium dioxide for photodegradations, under concentrated sunlight. That proves, expensive TiO2 can be replaced by inexpensive ZnO for photo-detoxification and disinfection of contaminated waters, under concentrated sunlight.
--------------------------------------------------------------------------------------------------------------
0* : Measurements of fluorescence of solutions kept with photosensitiser in a dark medium.
ACKNOWLEDGEMENTS :
We are thankful to Research Fund Organization of Ege University for financial support , Scientific Research Council of Turkey and State Planning Organization of Turkey ( DPT).
References:
3. S. Charterjee, S. Sarkar and S.N. Bhattacharyya, J. Photochem. & Photobiol. 72 (1993) 183.
4 . O. Legrini, E. Olivers and A.M. Braun, Chem. Reviews, March/April (1993) 671.
5. Richard, C., Boule, P., Aubry, J. M., J. of Photochem. Photobiol. A: Chem., 60, (1991), 235 – 243.