 doughnut-shaped
cyclic oligosaccharides.
doughnut-shaped
cyclic oligosaccharides. | Photoiupac home page | Discussion | Photobiology.com home |
Binding of a fluorescent coumarin derivative to b-cyclodextrin: a spectroscopic approach.
Régine Dondon and Suzanne Fery-Forgues
Laboratoire des
I.M.R.C.P.
Université Paul Sabatier
118, route de Narbonne
31062 Toulouse cedex
France e-mail : sff@gdp.ups-tlse.fr
Cyclodextrins (CD) are
naturally-occuring,  doughnut-shaped
cyclic oligosaccharides.
doughnut-shaped
cyclic oligosaccharides.
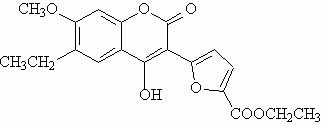
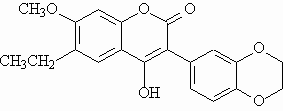
Fluorescent polysubstituted 4-hydroxy-coumarins 1 and 2 were used in this study as models for compounds to be vectorized.
Their properties in organic solvents were the topic of a previous work.5 Their optical properties were studied here in the presence of CD. Additional methods such as NMR were used, with the aim to relate the spectroscopic effect with the structure of the complex formed.
|
Solvent |
labs/nm/nm |
lem/nm/nm |
Ff |
t /ns |
|
1,4-Dioxane |
368 |
433 |
0.66 |
2.3 |
|
Ethyl acetate |
366 |
429 |
0.79 |
2.2 |
|
Dichloromethane |
368 |
425 |
0.17 |
1.1 |
|
Acetonitrile |
366 |
427 |
0.38 |
1.8 |
Attractive optical properties in organic medium:
- High quantum yield
- Lifetime in the ns scale
1 is poorly sensitive to variations of viscosity and temperature.
|
Solvent |
labs/nm/nm |
lem/nm/nm |
Ff |
t /ns |
|
1,4-Dioxane |
324 |
414 |
3.3 × 10-3 |
1.0 |
|
Ethyl acetate |
322 |
424 |
1.6 × 10-3 |
1.2 |
|
Dichloromethane |
328 |
414 |
2.3 × 10-3 |
0.7 |
|
Acetonitrile |
322 |
434 |
7.4 × 10-3 |
£ 0.7 |
- Lower quantum yield
- Shorter lifetime than probe 1.
The fluorescence properties of 2 are very sensitive to temperature and viscosity variations. Deactivation was attributed to the rotation of the benzodioxanyl substituent
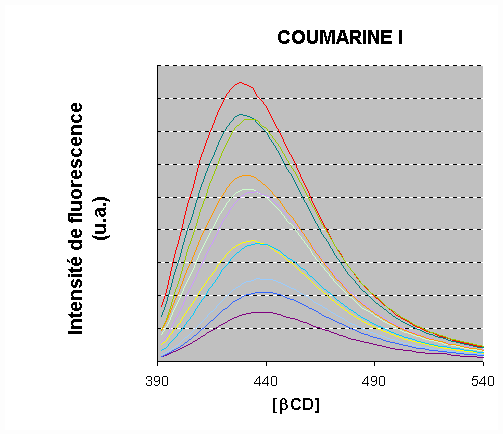 A strong increase of the fluorescence intensity was observed for 1 and 2 upon addition of
b-CD
A strong increase of the fluorescence intensity was observed for 1 and 2 upon addition of
b-CD
A blue-shift was observed in the presence of b-CD. The fluorescence intensity rose by a factor of 6.4 and 10 for 1 and 2, respectively.
An increase of the lifetime was also observed.
The emission data were processed.
A satisfactory fit was obtained by assuming a 1 :1 stoichiometry :
The binding constants were calculated:
coumarin |
K |
1 |
7 × 102 |
2 |
3 × 102 |
· In circular dichroism, a positive effect was observed, indicating incorporation.
Two different 1:1 complexes could be formed:
·NMR confirms incorporation and indicates which fragment of the coumarin is incorporated.
·The different spectroscopic methods used in this work confirm the incorporation of coumarins 1 and 2 in b-cyclodextrin. A 1:1 complex is most likely formed. This complex could be of type a (incorporation of the coumaryl cycle in the b-CD).
·This explains the blue shift observed in the fluorescence spectra, since the cavity of CD has been shown to be less polar than water.
·It also seems that incorporation induces a steric constraint on the probes substituent and interferes with the rotation, as suggested by the variation of the fluorescence properties which is comparatively stronger for 2 than for 1.
Cyclodextrins are increasingly used for drug delivery. Their toxicity is weak, and they can vectorize compounds of biological interest. The probes used in this study are models for natural coumarin derivatives which have recently been extracted6 from Triphasia trifolia (rutacea). This plant grows in the Caribbean, and is known in indigenous medicine for its anti-parasitic activity.
Triphasia Trifolia "citronnelle à épines"
Purification sometimes makes the drugs insoluble, because they are deprived of the molecules they are naturally associated with. Consequently, it is important to solubilize these drugs again.
In this study, solubilization reached 87% for 1 and 77% for 2 using 10-2 M b-CD and 10-5 M coumarin and it was limited by the solubility of CD. However, CD’s can also be chemically modified in order to increase their solubility in water.
5020-5022
New J. Chem. 1999, 23, 923-927.
Potier P., J. Nat. Prod., 1994, 57, 846-848.