PHOTOPRODUCTION OF ROSE OXIDE UNDER CONCENTRATED SUN LIGHT VIA SUPEROXIDE ANION RADICAL
Haluk Dinçalp and Sıddık IÁli
Department of Chemistry, Faculty of Science, Ege University,35100 Bornova, Izmir Turkey
Introduction
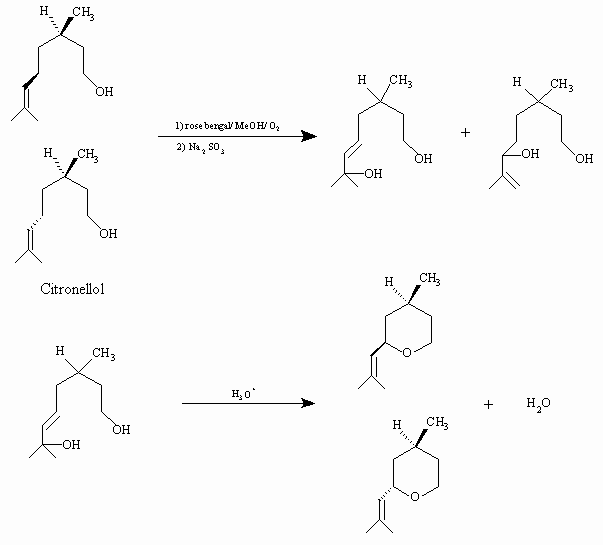
Synthesis of rose oxide, a valuable parfume additive, via singlet oxygen photooxydation of citronellol, under visible light, is proven by Schenk1. A triplet sensitizer rose bengal in methanol is being employed for photooxydation reaction, and hydroperoxide intermediates are reduced with sodium sulfide to form the isomeric diols. Diols are converted to isomeric rose oxide molecules by heating in an acidified solution.
Singlet oxygen photooxidation route to rose oxide is given below:

Scharf et al.2 reports the photoproduction of rose oxide under concentrated sunlight, 10-100 suns, by the employment of various triplet sensitizers fluorescein, eosine, erythrosine B, rose bengal, phenothiazine, methylene blue and porhyrins in numerous organic solvents. Photosensitizer was added beginning from 100 ppm to 5% by weight of the solution, and more addition was done in the course of the reaction. In general all of the organic photo sensitizers used, are photo unstable under sun light, and therefore additional input of sensitizer is required during the reaction. This obligation during the course of the reaction, is disadvantageous for bulk production, causes high consumption of the pigment and may arise problems on purification processes
 .
.
Figure: Bomin Solar automatic focused concentrator, in use at Ege University, Izmir, Turkey.
Perylene diimides
We now introduce a novel method for photoproduction of rose oxide under concentrated sunlight by the use of a photo and thermal pigment, perylene diimide. Perylene diimide is a singlet photosensitizer, and produces only super oxide anion radical at visible light excitation3. Absorbtion in the visible region (450-530 nm) enables them for use in solar applications. Energy and electron transfer processes proceed through singlet states. They are capable of reacting as an electron acceptor and as well as an electron donor. Fluorescence quantum yields of unity and high energy difference between singlet-triplet states, 54-27 kcal/mol, respectively, are taken as evidences to singlet state photo processes.
Perylene diimide
Experiments
Citronellol, 0.2M, in acetonitril, was irradiated in presence of 1-5 ppm perylene N-dehydroabietil diimide for 10 hours under 400 W sodium lamp photoreactor. Reaction was followed in t.l.c., product analysis was performed in GC-MS spectrometry system after sodium sulfide reduction and acidification. Results have revealed that rose oxide forms about in same ratio as by employment of rose bengal sensitizer. Same experiment was done under concentrated sunlight of 15-20 sun for 4 hours by the use of a Bomin Solar automatic focused concentrator(Figure). Product analysis has shown that rose oxide forms in similar yield.
Results and Discussion
Chen et al.3 reports the photooxidation of alfa- terpinene to p-cymene by the use of perylene diimide photosensitizer. It is known that perylene diimide forms only super oxide anion radical, through a singlet state electron transfer, and super oxide anion radical forms hydrogen peroxide by a hydrogen abstraction from the olefin. Formation of hydrogen peroxide is proven at photooxidation processes of perylene diimides3.
As seen on the scheme below, photosensitized oxygenation proceeds by an electron transfer from exciplex to oxygen, that results in formation of superoxide anion radical. Final product is rose oxide, by the hydrogen abstraction of hydroperoxyl intermediate.
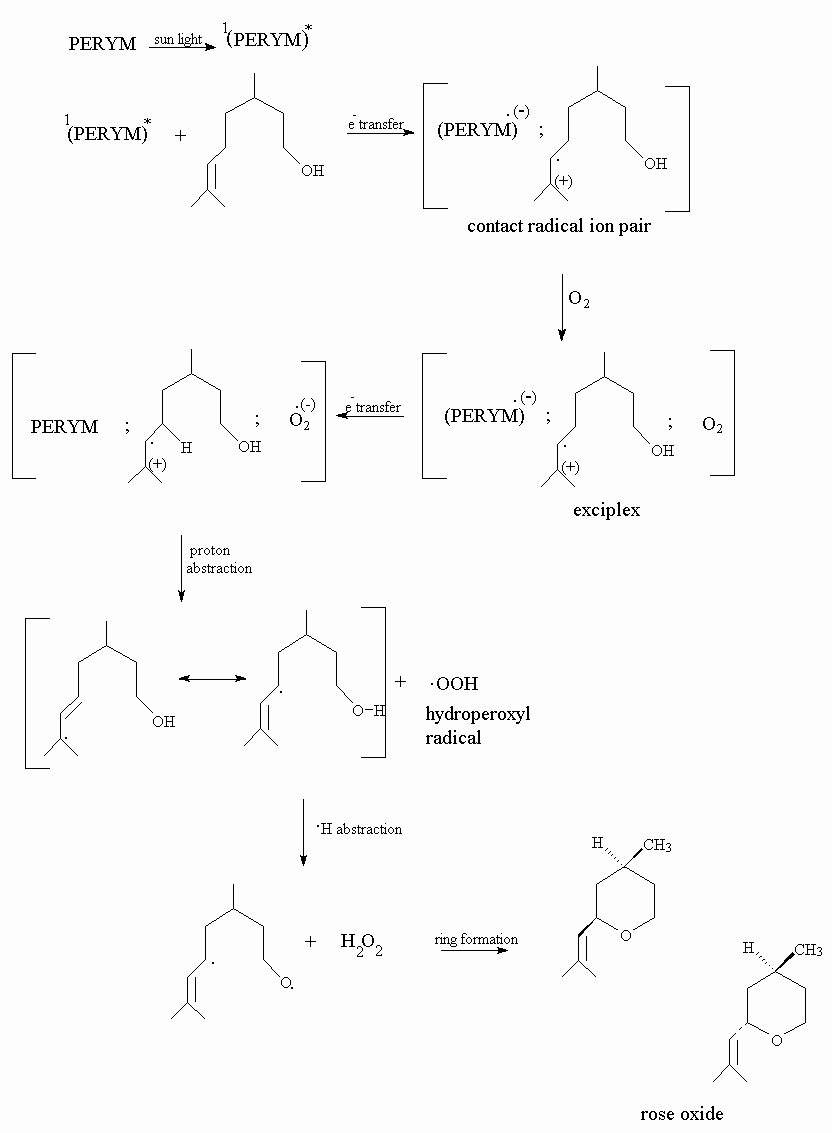
Perylene diimide do not degrade at all under high intensity of solar light and may be re-used by alcohol addition precipitation. It is expected that super oxide anion radical reaction with citronellol gives similar hydroperoxy intermediates as formed at singlet oxygen reaction. GC-MS spectrums show that rose oxide is produced at final step.
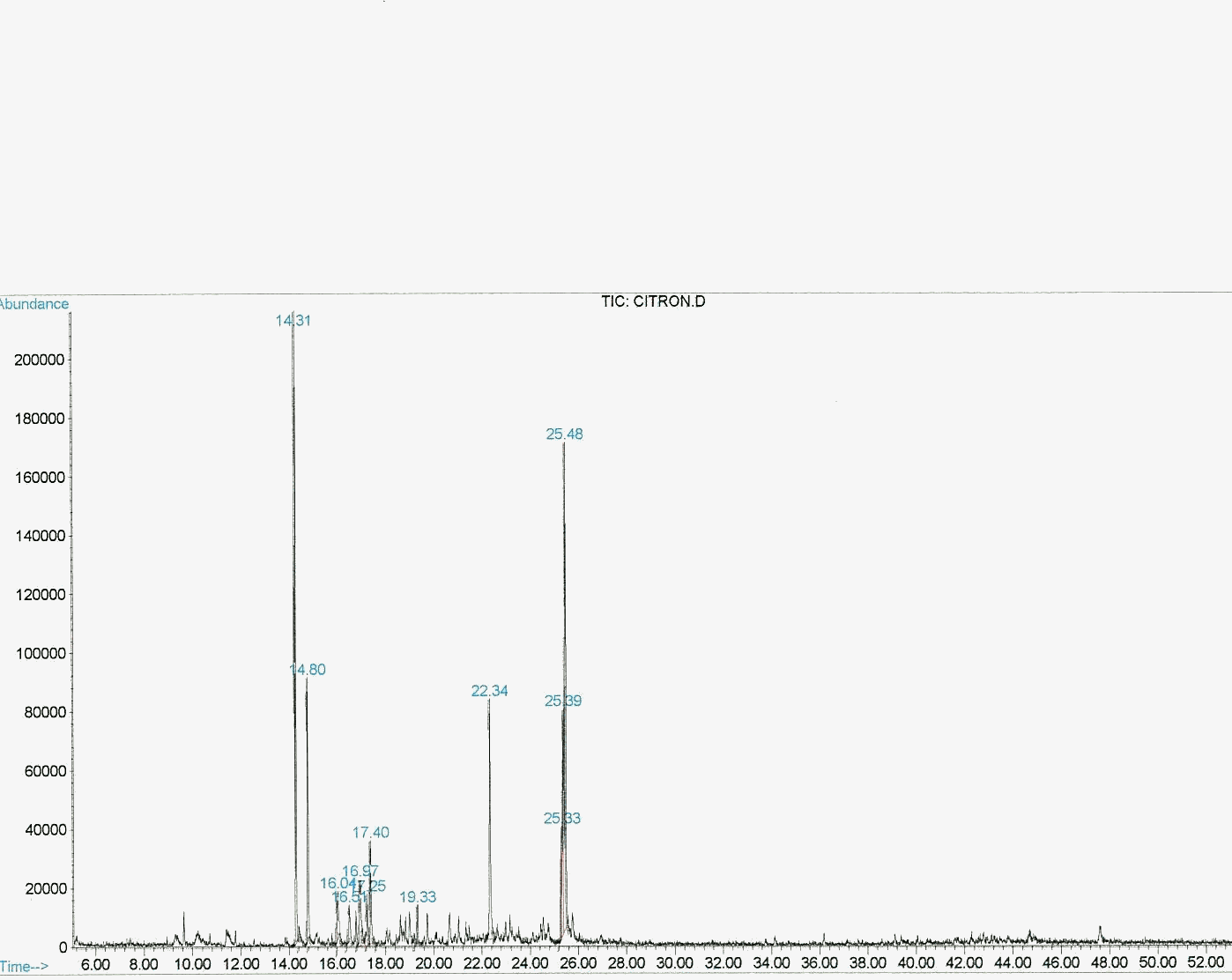
Graphic 1: GC spectrum of rose oxide synthesized under 400 W sodium lamp photoreactor
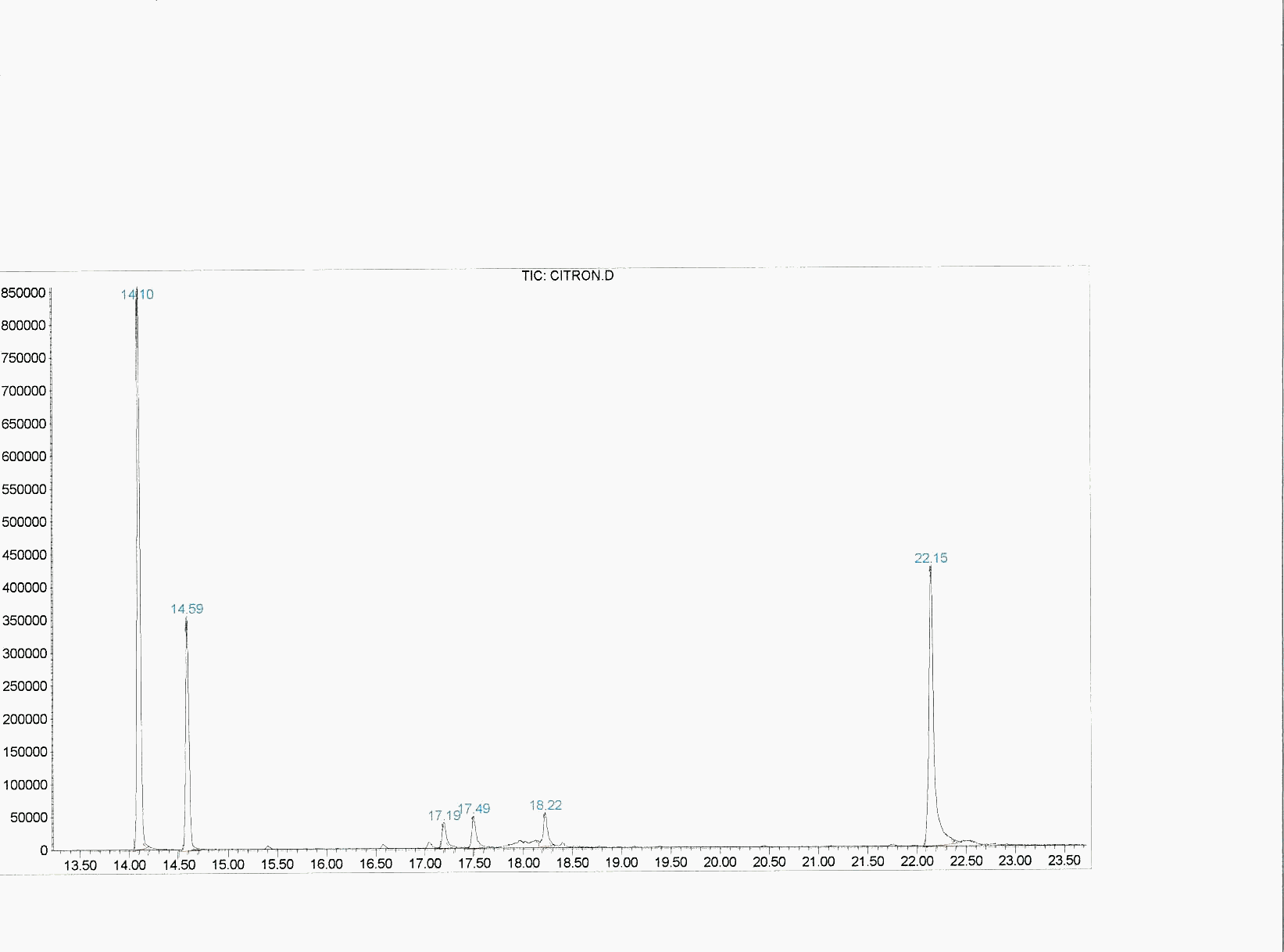 Graphic 2: GC spectrum of rose oxide synthesized under the concentrated sunlight
Graphic 2: GC spectrum of rose oxide synthesized under the concentrated sunlight
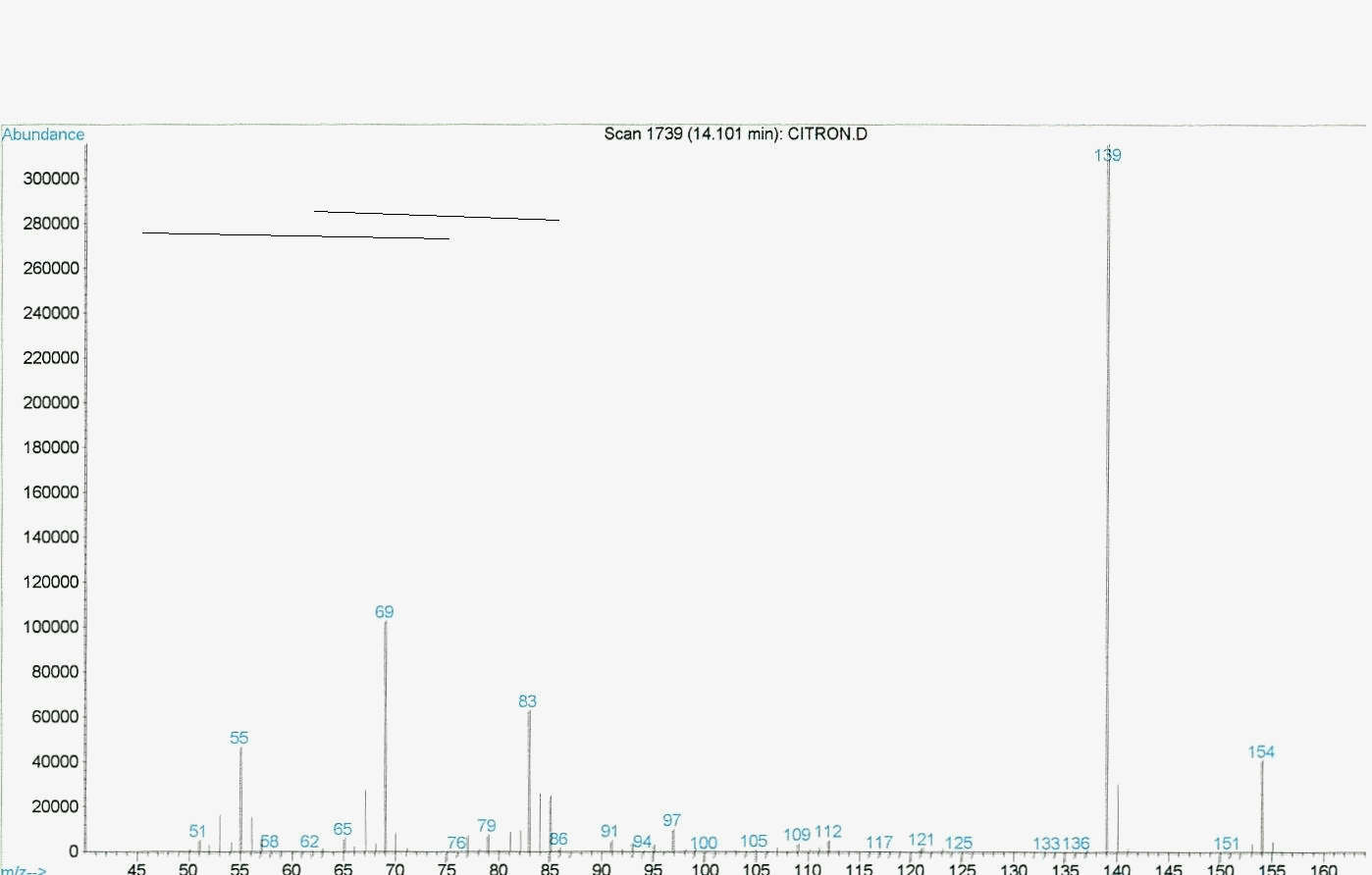
Graphic 3: MS spectrum of rose oxide synthesized under the concentrated sunlight
References
1. G. Ohloff, E. Klein and G. O. Schenk, Angew. Chem. 73 (1961) 78.
2. Patent US 1997 5,620,569, H.D. Scharf, P. Esser, W. Kuhn and R. Pelzer, Process for the photooxidation of terpene olefins, Haarmann & Reimer GmbH, april 15, 1997.
3. L. Chen, L.A. Lucia, E.R. Gaillard, H. Icil, S. Içli and D.G. Whitten, Jour. Phys. Chem. 102, (1998) 9095.