| Photoiupac home page | Discussion | Photobiology.com home |
OSCILLATIONS OF PHOTON EMISSION ACCOMPANYING THE OXIDATIVE PROCESS IN AQUEOUS SOLUTIONS OF GLYCIN WITH RIBOSE OR GLUCOSE
V.V. Koldunov, D.S. Kononov, and V.L.Voeikov
Dep. of Bioorganic Chemistry, Faculty of Biology, Lomonosov Moscow State University, 119899, Moscow E-mail: vvl@soil.msu.ruAbstract
Maillard reaction (MR) proceeding between reducing sugars and amino acids is known to be followed by weak chemiluminescence (CL). We have shown previously that CL may develop at room temperature after a short-term heating of an aqueous glycin/glucose solution. Here we demonstrate that under specific initial conditions oscillations of photon emission may arise in such reaction systems. Their amplitude may reach 20% of the average level of CL, and they endure for many hours. Duration and amplitude of oscillations depend upon reagents concentration, volume of the reaction samples, free surface area in contact with air and the presence of transition metal ions or ascorbate in micromolar concentration. The frequency spectrum of oscillations is more conservative. We speculate the existence of CL oscillations may be explained by oxygen-dependent chain reactions with delayed branching and consider the given oscillatory system is a suitable model for study of self-organisation processes.
Introduction
Most of the processes associated with free radical reactions are followed with ultra-weak or low-level chemiluminescence (CL). First reactions of this type were discovered by A. Gurwitsch, who observed that a brief irradiation of aqueous glycin and other amino acid solutions with a weak source of UV-light initiates the processes accompanied with mitogenetic radiation emission . Isolation of the reaction system from air halted emission, but after restoration of contact with air it immediately resumed. Gurwitsch suggested that energy for the emergence of electron excited states (EES) in this reaction system was drawn from oxidative reactions. In course of them free radicals arise, and electron excited products appear in reactions of their recombination. Relaxation of excited particles to ground state may be followed with photon emission. Alternatively, the energy of excitation may be transferred to certain fluorophores present in the system either directly, or non-radiatively and be emitted by them in another spectral region (sensibilized luminescence). Radical generation and recombination in such systems are very rare events, the quantum yield of photon emission in these processes is usually low, so the intensity of radiation is very weak . However, Gurwitsch and his school proved that despite of low intensity the emission was of profound biological significance: it is the necessary condition for the mitotic cycle initiation, and modulates many other cellular processes.
Unfortunately, this line of investigation wilted for a long time and restarted only when photoelectronic multiplier tubes were applied for the studies of lipid peroxidation in biological membranes and oils, and similar reactions in organic solvents . These reactions were considered to be purely degradative, and no biological function was ascribed neither to EES generation, nor to photon emission. However, the last assertion was shaken by Cilento, who discovered that photon emission arising in oxidative reactions catalyzed by peroxidases, serves as the energy of activation for some biochemical processes . Such reactions may proceed not only in lipid, but also in an aqueous phase ("photobiochemistry without light"). In recent years the number of newly discovered photoreactive enzymes and photon-generating processes was growing very fast .
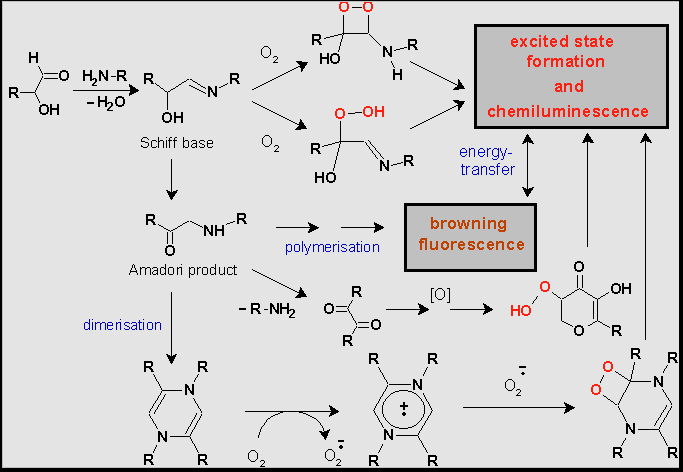
Not long ago it was shown that weak CL accompanies Maillard reaction (MR). MR is a complex set of different chemical transformations starting with condensation of the carbonyl group of reducing sugars and primary amino group of amino acids. In aqueous phase MR usually proceeds in several stages . First, Schiff bases (aldimines or ketimines) are formed. They spontaneously experience intramolecular Amadori rearrangement with the formation of ketoamines (Amadori products). Then Amadori products undergo conversions predominately by reactions of cleavage and hydrolysis yielding unsaturated products, which are strong reductants. They absorb in UV-range, and some of them have fluorescent properties. On the last stage dark-stained high molecular weight substances (0,2 –100 kDa) - melanoidins or advanced glycation end products (AGEs) - emerge as the result of polymerisation and polycondensation reactions. Under alkaline conditions a range of low molecular weight products arises (heterocyclic and acidic compounds) whereas polymers virtually are absent .
MR was initially the subject of food chemistry studies, because it accompanies thermal processing of food, the processes of wine and bear ripening and affects meals and drinks taste, aroma, and colour. Recently MR began to attract growing interest as playing an important role in ageing and pathogenesis of different diseases correlated to hyperglycaemia . Of particular interest is the ability of the products of MR starting with the Schiff bases to reduce molecular oxygen to superoxide anion radical (О2¾ · ) and other reactive oxygen species (ROS) . It was also shown that if MR is performed at high temperatures it is accompanied by weak CL . To explain the appearance of CL some potential pathways to electronically exited species have been proposed (Scheme 1). , .
It is supposed that in the course of MR dioxetane intermediates may arise owing to two-electron reduction of molecular oxygen. They decompose with the formation of triplet state carbonyl compounds. Besides, one-electron reduction of O2 occurs. As soon as О2¾ · appear, chain reactions may follow, giving birth to new free radicals and electronically excited products by reactions of their recombination . It is supposed by the analogy with the radical autoxidation of unsaturated fatty acids that in aerobic conditions free radicals can be maintained and even propagate by chain reactions with delayed branching . Indeed hydrogen peroxide and hydroperoxides, which can be the starting points to the branching are detected in the reaction system . Besides, stable and long-lived radicals appear due to the formation of heterocycles, which are able to give non-centered radicals at one-electron transfer 15. Photon emission arise when electron excited products relax to the ground state and also due to sensibilized luminescence of fluorescent compounds present in the reaction system 14.

Scheme 1: Suggested oxidative pathways in Maillard reaction leading to the formation of electron excited products and chemiluminescence
We have previously shown that after a brief heating of alkaline glycin/glucose solution and its cooling down to room temperature, a wave of CL gradually develops in the solution and photon emission lasts for many hours . Macro-kinetic properties of the process suggested that this reaction proceeded as a chain reaction with delayed branching and that self-organisation occurred in this system. In the present study we show for the first time, that this process may proceed as a classical Beloussov-Zhabotinsky reaction. Sustained, intense, and reproducible CL oscillations are observed in the course of MR. Effects of transition metal ions and ascorbate additions to reaction systems prove that the reaction belongs to the type of branched chain reactions dependent on oxygen free radical generation.
Materials and methods
All reagents used were of guaranteed grade. All solutions are prepared immediately before experiments using Milli-Q water, obtained by technology of Millipore, USA. Solutions of glucose (120 mM) or ribose (30 mM) in phosphate buffer (25 mM; pH 11,0) were kept in a water bath (92 oC) for 5 minutes and rapidly cooled down to a room temperature. The cooled solution was mixed with an equal volume of 120 mM glycin aqueous alkaline solution or 150 mM glycin solution in case of using ribose at pH 11,0. In some experiments aqueous solutions of CuCl2, FeSO4 or ascorbic acid were added to this mixture.
CL was determined in liquid scintillation counters Mark-II (Nuclear-Chicago, USA) equipped with photomultipliers EMI 9750QB/1 (GB) (photocathode S-11; wavelength region 300-600 nm) in the single photon rate counting mode. Reaction mixtures in volumes from 2 to 20 ml were poured in standard 20-ml borosilicate glass vials for LSC or in glass test-tubes, installed in the same vial. The vials were held stationary in a counting chamber. CL was registered at room temperature with high temporal resolution (0,1 or 0,2 min) for prolonged periods of time. Fourier analysis was performed by means of the program package Statistica 5.0.
Results
CL development in glucose(ribose)/glycin solutions, caused by heating of the monosaccharide solution and followed with addition of a cold glycin solution has remarkable properties. As is seen from Fig. 1,A (curves 1 and 2) three macrokinetic stages may be distinguished: the relatively short stage of CL intensity declining from initially high values, enhancement of CL intensity, and the stage of its gradual fading away. Previously we designated the last two stages as a CL-wave 22. In the absence of glycin CL intensity decreases monotonically (curve 3). Similar behaviour was observed for CL kinetics if the reaction in the presence of both amino and carbonyl compounds was proceeding in anaerobic atmosphere (curve 4).
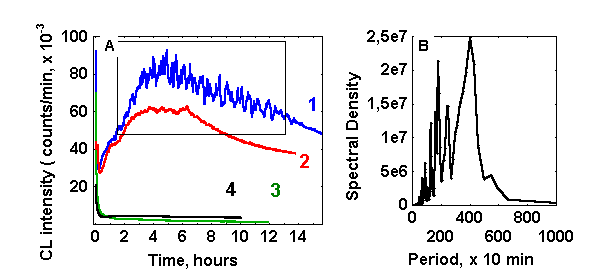
Figure 1: (A) Chemiluminescence from various reaction systems (10 ml, pH 11,0): (1) glucose-glycin-ascorbate (60 mM, 60 mM, 100 mkM, respectively); (2) glucose-glycin (60 mM each); (3) glucose (60 mM); (4) glucose-glycin (60 mM each, bubbled with argon and isolated from air). (B) Fourier spectrum of the fragment of the curve 1 (plot A), inserted in a box.
As a rule, oscillations of photon emission start at the stage of CL rise and disappear at some point after the maximal CL intensity has been reached. Dependent on the experimental conditions CL oscillations lasted from several hours to 2-3 days. The amplitude of oscillations reached sometimes values of 20 % of the average values of CL intensity. Fourier analysis (figure 1,B) revealed that dominating periods of oscillations are in the range of 15 – 40 min, while shorter periods have much lower spectral density.
Noteworthy is also a surprising similarity of oscillatory patterns in the repeated experiments if the conditions of sample preparation were kept as similar as possible. Fig. 2 demonstrates fragments of CL dynamics in two such samples prepared in different days. Dissimilar time scales on these plots reflect that alike oscillatory patterns were developing in the two samples with different overall rates, probably because of some differences in the ambient temperature on these two days.
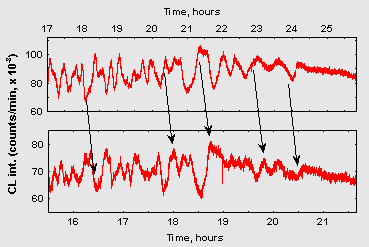
Figure 2: Fragments of chemiluminescence dynamics from two experiments (10 ml 15 mM ribose/75 mM glycin, pH 11,0) prepared on two different days. Abscissa – time from the start of CL registering. Arrows denote similar patterns in two curves.
As it can be seen in Figure 3, A, oscillations in CL intensity are poorly manifested in a solution containing only glucose and glycin (curve 1). Addition of CuCl2 to the reaction mixture at concentration as small as 0,1 mkM resulted in pronounced oscillations. At higher salt concentrations, oscillations emerged earlier, the amplitude and time of their duration increased, though the frequency characteristics of oscillations remained essentially unchanged. In the presence of CuCl2 CL intensity was initially lower, but then became higher than in control. Analogous results were obtained if FeSO4 was added to the reaction mixture, with the difference, that ferro ions exerted the same action as copper in concentration roughly 2-3 orders of magnitude higher (figure 3, B).
When glucose was replaced with ribose pronounced oscillations of CL intensity were registered even in the absence of transition metals (figure 4). Addition of 1 mkM CuCl2 changed macrokinetic properties of photon emission in the same manner as in glucose/glycin systems. Same maximal CL intensity and amplitude of its oscillations were observed at much lower ribose, than glucose concentrations. However, Fourier analysis of oscillations in ribose/glycin solutions did not reveal any significant changes in spectral properties of oscillations.
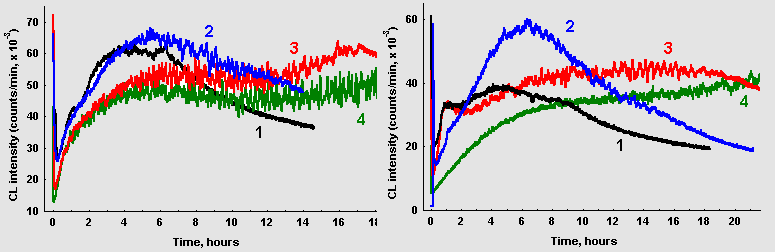
Figure 3: Effect of transition metals ions on CL from glucose-glycin systems (60 mM each, 10 ml) A: (1) control; (2) 0,1 mkM CuCl2; (3) 1,0 mkM CuCl2; (4) 10 mkM CuCl2. B: (1) control; (2) 0,01 mM FeSO4; (3) 0,1 mM FeSO4; (4) 1,0 mM FeSO4.
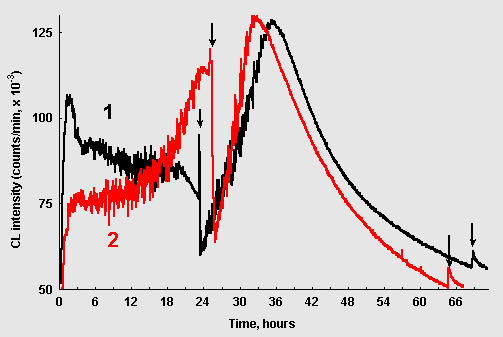
Figure 4: Chemiluminescence from ribose-glycin systems (15 mM / 75 mM, pH 11,0; 10ml): (1) without CuCl2; (2) 1,0 mkM CuCl2. The arrows point to the moments when 10mkl of CuCl2 solution (10mM) were added.
If CuCl2 was injected into the ribose/glycin reaction at the stage when CL was fading out, CL intensity first sharply dropped down, and then CL began to grow again in an oscillatory mode (Fig. 4, curve 1). It reached maximal values exceeding those observed during the first "wave" of CL. The similar picture was observed when CuCl2 was introduced into the system where it was initially present at concentration of 1 mkM (Fig. 4, curve 2). When the reinitiated wave of CL faded out new addition of copper resulted only in a slight and transient CL spike (arrows in the right bottom corner of Fig. 4).
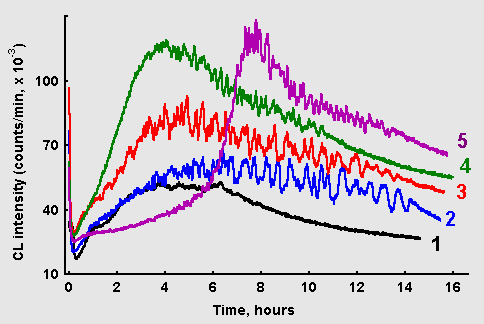
Figure 5: Chemiluminescence from glucose-glycin systems (60 mM each, 10 ml) in the presence of ascorbate: (1) without ascorbate; (2) 1 mkM ascorbate; (3) 10 mkM ascorbate; (4) 100 mkM ascorbate; (5) 1 mM ascorbate.
Effects of ascorbate addition to the glucose/glycin reaction mixtures on CL kinetics is illustrated in Figure 5. Rather complex dependence of ascorbate action on its concentration can be seen. Ascorbate in concentration of 1 mkM does not affect the rate of CL development at the early stage, but as the CL intensity approaches maximum values, prominent oscillations appear and continue for many hours. Besides, CL with ascorbate fades out much later than in the control reaction. At higher ascorbate concentrations (10-100 mkM) CL increases at a higher rate, and its maximal values become higher. However, at the highest tested ascorbate concentration (1 mM) a long-lasting latent period in CL development revealed itself, after which CL sharply increased with pronounced oscillations.
Dependence of CL kinetics in glucose/glycin solutions on reaction system volumes and on the area of the solution surface contacting with air are illustrated in Figure 6. Data presented in the left frame were obtained when the volume was changing at the constant surface area, and data in the right frame describes CL evolution in samples with two different volumes, but the same surface-to-volume ratio. As the reaction volume increases, maximum of CL intensity shifts to the right and the rate of CL development slows down but oscillations become more prominent.
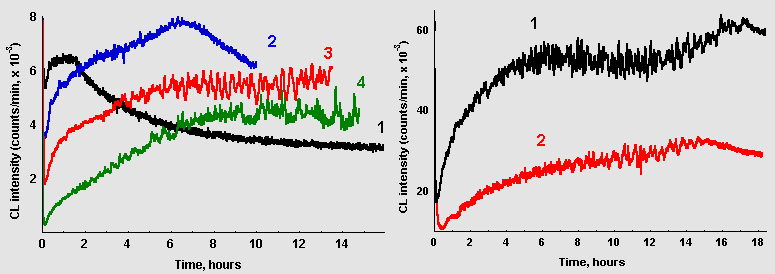
Figure 6: A. Dependence of CL from glucose-glycin systems (60 mM each, pH 11,2) on sample volume: (1) 2 ml; (2) 5 ml; (3) 10 ml; (4) 20 ml (data recasted per 1 ml of the solution) B. CL from the same reaction systems poured in vessels with different surface area: (1) 10 ml, reservoir diameter 25 mm; (2) 4 ml, reservoir diameter 14 mm.
However, oscillations could emerge in solutions with small volumes if the area of the solution contacting with air was reduced. As it can be seen in Fig. 6, B, marked oscillations are observed in 4 ml of the reaction mixture when its surface area is reduced more than 3 times comparing to previous experiments.
Discussion
Previously we found that after brief heating of alkaline glucose/glycin solution and its cooling down the long-lasting wave of CL develops in it. Some features of CL kinetics under different conditions indicated that chain reactions with delayed branching could proceed there though the evidence in favour of this mechanism was not enough 19. Here we modified the procedure of the sample preparation. Only alkaline sugar solutions instead of sugar/glycin solution are heated, and glycin is added to already cooled down sugar solution. This allowed to distinguish better different stages in the process giving birth to photon emission, to make more valid conclusions about the mechanism of the reaction, and also to discover a new phenomenon – prominent and long-lasting oscillations of CL in sugar/glycin reaction mixtures.
Initial CL level in a heated-cooled alkaline sugar solution is very high, and it fast goes down and stays at a low quasi-stationary level for an indefinite time. We consider, that the energy source for photon emission here is recombination of free radicals emerging in the oxidative reactions of aldehydes and relative substances (the latter are formed in heated alkaline glucose solutions). Oxidative reactions of aldehydes are known to proceed usually as linear chain reactions (pp. 299-305). In such reactions concentration of active centers (free radicals) gradually declines due to chain breaks. Hence if events of radicals recombination are the major source of electron excited states (EES) formation initially high photon emission must gradually decline. However if the sugar solution was mixed with the glycin solution the second long-lasting wave of CL appeared. It developed even if the latter was added to the former many hours after heating cooling the sugar solution.
Macrokinetic picture of CL development after glycin addition to glucose or ribose solutions as well as some other properties of the process to be discussed later suggest that chain reactions with delayed branching take place here. N. Semyonov, who discovered of such reactions, suggested that they differ from usual branched chain reactions by formation of metastable intermediate products during the major chain propagation. Much lower energy of activation is needed to produce new active centers from such products than from the initial substances. Thus, even if the "parent" chain is eliminated, new chains arise in the system provided that enough energy for the activation of these metastable products is available. Such reactions are named "degenerate-branched chain reactions," though referring to them as to "Chain Reactions with Delayed Branching" seems to be more appropriate 21, p. 488. Evidently, in monosaccharide/glycin reaction mixtures one-electron reduction of molecular oxygen takes place, and just as О2¾ · appear, other reactive oxygen species (hydrogen peroxide, hydroperoxides, hydroxyl radical, alkoxyl radicals, peroxyl radicals) easily emerge. As it was mentioned in the Introduction, hydrogen peroxide and hydroperoxides, which in the case of their decomposition can be the starting points to the branching, had been in fact identified in reaction systems where MR proceeds. But a certain supply of energy of activation is needed for their decomposition. We suppose, that in the particular combination of reaction conditions, the energy of EES may be used in our system as the energy of activation for such metastable products decomposition with the arousal of new free radical. However, as the reaction "matures", more and more complex and even polymeric compounds emerge, which absorb and dissipate energy of EES relaxation, and/or act as chain breakers turning into stable radicals. The latter may be looked upon as strong antioxidants. With their accumulation in the reaction system CL fades out.
Experiments with ascorbate – the natural antioxidant – convincingly support the suggestion that the reaction studied here proceeds according to the mechanism of chain reactions with delayed branching. Ascorbate reacts with free radicals, forming the stable ascorbate semiquinone and therefore terminates chains of radical processes , . In 1 mM concentration ascorbate retards CL development, though after some time its intensity rapidly increases. Such a phenomenon is characteristic for the action of the so called "negative catalysts". In low concentrations relative to concentrations of major reagents they turn a non-stationary accelerated branched chain into a stationary one. So they prolong the induction period of branched chain reaction, but when a negative catalyst is consumed the active centers start to multiply and the reaction rate accelerates.
However, ascorbate in lower concentrations (1-100 mkM) seems to act as a prooxidant, because it accelerates CL development and augments its maximal intensity. In fact it has been shown that ascorbate may fulfil the role of prooxidant , though the mechanisms of prooxidant action of ascorbate are far from being clear. The effects of small and relatively high concentrations of CuCl2 and FeSO4 upon macrokinetic properties of the reactions are also opposite and in general similar to those of ascorbate. It is remarkable that in the range of high concentrations they behave here as antioxidants. These effects are paradoxical in view of the classical approach to transient metal ions as to pure prooxidants.
The ability of copper to reinitiate CL in the faded out solution is another argument in favour of the chain reaction mechanism with delayed branching. It was shown long ago that in the classical reactions of this type, such as a polymer oxidation, the reaction may practically stop long before the substrate is oxidised. The reaction propagation is blocked by some of its products with strong antioxidant properties, that are able to scavenge active centers. But, surprisingly, addition of certain other antioxidants to the reaction system could reinitiate oxidation. It turned out that at certain ratio of "weak" antioxidants to the "strong" ones, the former supported decomposition of metastable intermediate products, in particular, peroxides, with the generation of the new active centers . Reinitiating action of copper may be also explained by its ability to decompose peroxides. Finally, the dependence the dynamics of CL upon the volume and surface of contact with air also argues of in favour of the branched chain mechanism.
We consider that the discovery of sustained oscillations of CL intensity is the most interesting part of present study. There are only few reports in the literature about chemical reactions accompanied with oscillatory or pulsed CL. So called "bursts of cold fire" had been revealed in the course of oxidative branched chain reactions proceeding in the gaseous phase . As regards the aqueous phase, CL oscillations were registered in the classical Beloussov-Zhabotinskii reaction , and the reaction of NADP-H oxidation by peroxidase. Oscillations emerging in the course of MR are well pronounced, persist for many hours and are characterised by periods lasting for tens of minutes. Dependence of the oscillation emergence on the surface area of the reaction mixture suggests that one of the critical factors for them is the particular range of rates of oxygen diffusion into the solution containing oxygen-reducing substances. Another critical condition is the presence of "catalysts" of oxidative processes, such as transition metal ions, or ascorbate.
We suppose that in general two opposite processes occur in our system: 1) the synthesis of compounds capable to reduce oxygen and thus to support radical chain reactions initiation; 2) the synthesis of substances with the more or less strong antioxidant properties. Besides, intermediate metastable products accumulate, that may be starting points for chains branching. Due to the process #1 free radicals accumulate, and reactions of their recombination provide energy for the emergence of photon emission. Due to the process #2 radicals are scavenged, chains are broken, and photon emission diminishes. When conditions are disadvantageous for the oscillations emergence rates of the two processes change smoothly: at first the process #1 dominate and CL intensity grows, then the products of the process #2 build up and CL fades out. The overall CL is the result of relative contribution of each of the two processes. However, within the specific range of the rates of oxygen diffusion into the solution, and in the presence of the tuners of oxidative/reductive kinetics, such as ascorbate, conditions for the non-linear patterns of the two reactions proceedings may be established, and CL oscillations may develop. When the process #1 dominates, CL intensity increases, but at the same time O2 is being consumed and the reaction system becomes deficient of it. Under such conditions the process #2 takes over, and CL goes down. At the same time metastable products, such as peroxides, as well as oxygen reductants accumulate. When new portions of oxygen diffuse into the reaction system, it is reduced by the newly emerged reductants, free radicals level and the probability of the reactions of their recombination increases. The latter support energy of activation for the metastable products desintegration and emergence of new radical chain reactions. All this follows with a sharp burst of photon emission, which then inevitably expires due to exhaust of oxygen in the reaction volume. It is possible that other positive as well as negative feedback between different elementary steps and chains arise. At the current stage of our investigation the presented mechanism should be considered as a working hypothesis.
Finally, the high reproducibility of oscillation patterns in case of seemingly stochastic branched chain processes (Fig. 2) shows that there are very strict constraints on the course of the process under reasonably wide experimental conditions. Thus there is good reason to belief that the described system can serve a convenient working model for the studies of self-organisation processes taking place in an electronically excited milieu. The oscillations revealed here can shed light upon many problems, emerging in a frame of ‘biochemistry without light’ and shake currently prevailing opinion about extremely harmful role of ROS on living systems, based on the erroneous statement of the non-controlled nature of branched chain processes.
References