| Photoiupac home page | Discussion | Photobiology.com home |
PICOSECOND ANISOTROPY OF THE TRANSIENT ABSORPTION OF THE PHOTOCHROMIC MERCURY DITHIZONE COMPLEX IN SOLUTION
Barbara PACI and Jean-Michel NUNZI,
LETI (CEA - Technologies Avancées), DEIN/SPE Groupe Composants Organiques,
CEA Saclay, F-91191 Gif-sur-Yvette Cedex, France
Nadejda SERTOVA and Ivan PETKOV*,
University of Sofia, Faculty of Chemistry, Department of Organic Chemistry,
1 James Bourchier Av., 1126 Sofia, Bulgaria
Abstract
A picosecond Kerr ellipsometry study of the transient anisotropy of the metal dithizonate Hg(HDZ)2 in solution with different organic solvents is presented. The Hg(HDZ)2 photochromic complex is one of the first organic dyes shown to exhibit photochromic proprieties. The complex exhibits reversible photochromism, changing from the normal form A (of orange-yellow colour) to the active form B (of blue colour) after excitation with 532nm laser light. The Kerr ellipsometry signal is observed to decrease with a single exponential decay of about 100ps, that is shown to be independent of the solvent. This indicates that the signal is dominated by the excited state lifetime of the molecule. The absence of any spectral signatures relevant to the excited states of the normal form indicates that formation of the photo-isomer is a fast process with respect to the lifetime of the exited state.
*Corresponding author
E-mail: petkov@ortolan.cea.fr ; ipetkov@chem.uni-sofia.bg
INTRODUCTION
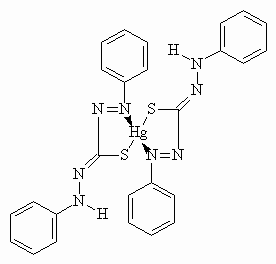
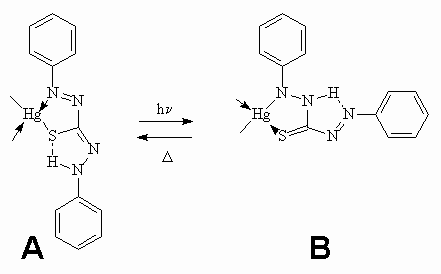
Metal dithizonates form an interesting class of photochromic complexes, being one of the first organic dyes to exhibit photochromic proprieties. Easy to synthesise, they form strongly coloured complexes with a large number of metals and are compatible with a variety of different media (solvents, host-guest systems, polymers, glass surfaces, etc.). In particular, mercury(II)bisdithizonate, Hg(HDz)2, has been deeply studied, since it has been shown to exhibit reversible photochromism in organic solvents [1-3]: the normal form A, of orange-yellow colour, changes to an active form B, of royal-blue colour, if irradiated by visible light [4-6]. This photochromic cycle can be repeated several times. The structure of the normal form, for the solid state and the ground state in solution is shown in Figure 1(a), together with the photochromic transformation involving a trans-cis isomerization about the C=N bond and N-to-N hydrogen transfer, Figure1(b) [5].

a
b
Figure 1. Structure of the Hg(HDz)2 molecule : the ligand forms an N,S-chelate ring with the metal atom (part a). Irradiation of the complex induces photochromism and the orange (normal) form changes colour to the blue (active) form (part b).
The normal form is converted by a photochemical trans-cis isomerization about N=N double bond into the isomer B which shows a lower absorption intensity at longer wavelength. The reason for the comparatively long-wavelength absorption of B might be a torsion about the C-N single bond. Form B may revert photochemically as well as thermally to the original form. A study of the colour response range induced by irradiation with visible light of Hg(HDz)2 in different solvents will be reported elsewhere shortly. In this paper, we study the transient behaviour of Hg(HDz)2 on a picosecond timescale following resonant optical excitation.
EXPERIMENTAL
Preparation of the samples
Hg(HDz)2 has been synthesised and purified following the procedure described in [4]. The samples consist of a fused quartz cell (d=0.1cm) filled with a solution of the molecule in different solvents : benzene, dichloro-1,2 benzene and 2-methoxyethyl ether. The respective viscosities and polarities of the solvent are reported in table I. All investigated compounds show absorption bands in the visible region (figure 2a).
For each solvent, a typical sample concentration of 3.9x10-5 mol/l results in an optical density close to 0.15 at 532 nm.
Linear absorption spectroscopy
UV-visible absorption spectroscopy has been performed in order to characterise the linear optical properties of the molecule and the absorption spectra, recorded on a Perkin-Elmer 19 UV/VIS spectrophotometer, are shown in Figure 2a.
|
a) |
|
|
b) |
|
Figure 2. Absorption spectra of Hg(HDz)2 in benzene (dashed line); 1,2-dichlorobenzene (dotted line); 2-methoxyethyl ether (continuous line), for c=3.910-5 mol/l (part a); Photochromic spectral changes of Hg(HDz)2 in benzene (c= 3.910-4 mol/l) due to visible light irradiation (part b).
The molar extinction coefficients at the wavelength of the laser excitation, obtained by the Beer-Lambert law, are listed in Table 1.
Table 1.
Linear optical properties of Hg(HDZ)2 in solution:
n is the solvent viscosity, P is the solvent polarity (relative to water), e is the molar extinction coefficient at 532nm and eA is the molar extinction coefficient at maximum absorption.|
Solvent |
n (cp) |
P (103 in water) |
e (l 532nm)((mol/l)-1cm-1) |
e A((mol/l)-1cm-1) |
||
|
benzene |
0.66 |
111 |
32051 |
69230 |
||
|
1,2-dichlorobenzene |
1.30 |
225 |
32051 |
58974 |
||
|
2-methoxyethyl ether |
1.90 |
244 |
32051 |
71794 |
||
The photochromic behaviour of Hg(HDz)2 under irradiation with visible light is related to the effectiveness of the isomerization processes depending on the characteristics of the solvent used. In the case of solutions in benzene and in 1,2-dichlorobenzene a change in the colour of the sample is observed after irradiation, but only for benzene’s solution the relaxation to normal form is sufficiently slow to allow a measurement of the linear spectrum of the activated form B. In the case of the solution with 2-methoxyethyl ether, on the contrary, no evidence of isomerization is observed. The reason for this may be the formation of hydrogen bonds with the solvent that inhibit the photo-process. Isomerization is activated by irradiating with visible light above 420nm, in the region of the visible absorption. Figure 2b shows the spectral changes in the absorption spectrum of Hg(HDz)2 in benzene (c=3.9 10-4 mol/l) due to visible light irradiation. The spectrum shows the appearance of a new absorption band in the 550-700 nm region after irradiation with visible light. This band is attributed to the coloured B form in Figure 1b. An isobestic point is clearly visible at 530nm. Both solutions with benzene and 1,2-dichlorobenzene do not show fluorescence when exited with 480nm light, while solution with 2-methoxyethyl ether exhibits a week fluorescence near 550nm.
Nonlinear spectroscopy
Kerr ellipsometry measurements have been performed at different time delays. The experimental set-up for picosecond Kerr ellipsometry is described in ref. [7]. Nonlinear optical Kerr ellipsometry is a pump-probe technique allowing the separation of the real and imaginary part of the photoinduced anisotropy. A frequency doubled Nd3+:YAG laser (532nm, 32 ps) is used as the pump beam and a continuum, generated by focusing part of the fundamental laser beam in a deuterated water cell, is used as the probe beam. Pump fluence at the sample is typically 5.6mJ/cm2. The time delay between the two beams is adjusted from 100ps to 1.5ns using a variable delay line. Time zero is defined in correspondence with pump-probe overlap. The sample is placed inside a Kerr gate composed of two perpendicular polarisers. After interaction inside the sample, the probe beam is dispersed by a spectrometer coupled to a CCD camera. The pump beam, with strong intensity, induces transient birefringence and dichroism in the initially isotropic sample. The probe beam is initially linearly polarised at 45° to the linear polarisation of the pump beam. The induced anisotropy results in a change of the probe beam polarisation after interaction within the sample. This change is recorded for each wavelength of the continuum. Intensity measurements are averaged over 120 shots for each angle of the analyser.
In particular, measurement of the dichroic angle df (i.e. imaginary part of the induced anisotropy) allows a direct determination of the induced dichroism (difference of absorption coefficients between two perpendicular directions). The spectral dependence of df is directly related to that of the absorbance, while its time dependence provides information both on the excited state relaxation dynamics and on the molecular orientational diffusion inside the solvent.
RESULTS AND DISCUSSION
The Kerr-ellipsometry signal resulting from one photon excitation at 532nm can be attributed to the excited state absorption features of the molecules [8]. Notice that in the present case, excitation is performed close to the isobestic point between A and B forms. Practically, it is dominated by bleaching of the main absorption band, corresponding to the linear absorption of the normal form at 490nm. In more detail, in the case of solutions with benzene (Figure 3a) and 1,2-dichlorobenzene (Figure 3b) the Kerr spectra are characterised by 3 main spectral features : bleaching of the absorption band at about 490nm, corresponding to the linear absorption of the A form, bleaching of the absorption band at about 610nm, corresponding to the linear absorption band of the B form, and photoinduced absorption at about 720nm.
|
a) |
|
|
b) |
|
|
c) |
|
Figure 3. Dichroic spectra of Hg(HDz)2 in solution with benzene (part a), 1,2-dichlorobenzene (part b) and 2-methoxyethyl ether (part c). Spectra are recorded at 0, 133 and 266 ps time delay after 532nm excitation. Base line is shifted upward for clarity. The sharp peak at 532nm is due to pump scattering.
In the case of the solution in 2-methoxyethyl ether, only a strong signal from the bleaching of the main absorption band at 490nm is visible (Figure 3c), indicating the absence of any signature of the B form. This confirms that with this solvent, the B isomer is not formed upon optical excitation. As a consequence, photoinduced absorption at 720nm in benzene and dichlorobenzene (Figures 3 a and b) may be attributed to the excited states of the B form.
For all solutions, the Kerr spectra recorded at longer time-delays present the same profile as the one observed at zero-delay. The signal is observed to decrease rapidly, with a monoexponential decay time
t. Table 2 contains the values obtained for the normal and activated forms : tA and tB being decay times of the signal at lA and lB, together with the Kerr ellipsometry absorption cross sections calculated at lA and lB for the different solutions.Table 2.
Nonlinear spectroscopic data measured by Kerr-ellipsometry :
lA (lB) is the wavelength of the Kerr peak relative to bleaching of the absorption band of the A (B) form; dfA (dfB) is the dichroic angle at lA (lB); sA (sB) is the absorption cross section at lA (lB) measured by Kerr ellipsometry; tA (tB) is the decay time of the Kerr signal amplitude at lA (lB) and torr is the orientational decay time deduced from the Debye-Stokes-Einstein formula : torr = hV/kT where V is molecular volume (estimated to 1436 Å3), h is solvent viscosity and kT is temperature.
|
Solvent |
lA / lB(nm) |
dfA / dfB(10 -2 rad) |
s A / sB(10-17cm2) |
t A / t B(ps) |
t orr(ps) |
|
benzene |
490 / 604 |
0.20 / 0.23 |
0.58 / 0.67 |
146 / 115 |
238 |
|
1,2-dichlorobenzene |
490 / 604 |
0.30 / 0.14 |
0.88 / 0.40 |
103 / 96 |
684 |
|
2-methoxyethyl ether |
490 |
0.70 |
2.05 |
120 |
468 |
Measurements were performed using solvents with different viscosities and no increase of the time constant with solvent viscosity is observed, indicating that molecular orientational diffusion is not the major cause of signal decay. Orientation decay times
torr deduced from the Debye-Stokes-Einstein formula are given in Table 2 : they are larger than the measured Kerr signal decay times. This shows that signal decay is dominated by the excited state lifetime of the molecule. Additionally, as no signature of the excited states of the A form is observed in Figure 3, we can conclude that formation of the photoisomer B is a very fast process which occurs during recovery of the A form bleaching: that is typically within 100ps in all the solvents investigated.For all solvents, bleaching cross sections of the A form, sA measured by Kerr ellipsometry at lA (Table 2) are one order of magnitude smaller than the one obtained from the extinction coefficients eA measured by linear absorption (Table 1); they are related by eA = NAsA/ 103Ln10 where NA is Avogadro number, yielding absorption cross sections in the 2.5x10-16cm2 range for all 3 solvents. This apparent discrepancy may be essentially attributed to the rather low anisotropy of Hg(HDZ)2 which is a non planar molecule with qualitatively, a more spherical-like than rod-like shape. Indeed anisotropy which is maximum for rod-shaped molecules is vanishingly small for spherical-shaped molecules [9].
CONCLUSION
The anisotropy of Hg(HDZ)2 in solution with different solvents has been studied by picosecond Kerr ellipsometry. The Kerr signal is dominated by bleaching of the band corresponding to the linear absorption of the normal form at 490nm. In the case of solutions with benzene and dichloro-1,2benzene, two other bands appear : bleaching of the absorption band of the B form at about 610nm and photoinduced absorption of the B form at about 720nm. On the other side, there are no spectral signatures of the excited states of the A form, indicating that formation of the photoisomer is a fast process with respect to the lifetime of the excited state, of the order of 100ps. Experiments have been performed exciting both A and B forms, as pump laser wavelength was 532 nm, so that further experiments aiming at excitation of the B form only are in progress, in order to investigate in more detail the reverse photochromic transformation.
REFERENCES
[1] H. Irving, G. Andrew, E.J. Risdon, J. Chem. Soc., (1949) 541.
[2] J.L. A. Webb, I.S. Bhatia, A.H. Corwin, A.G. Sharp, J. Am. Chem. Soc., 72 (1950) 91.
[3] N. Sertova. I. Petkov, J.-M. Nunzi, J. Photochem. Photobiol. A: Chemistry 134 (2000) 163.
[4] L.S. Meriwether, E.C. Breitner, C.L. Sloan, J. Am. Chem. Soc., 87 (1965) 4441
[5] L.S. Meriwether, E.C. Breitner, N.B. Colthup, J. Am. Chem. Soc., 87 (1965) 4448.
[6] A.T. Hutton, H.M.N.H. Irving, J. Chem. Soc. Dalton Trans., 11 (1982) 2299.
[7]
N. Pfeffer, F. Charra, J.M. Nunzi, Opt. Lett., 16 (1991) 1987.[8] S. Delysse, J.M. Nunzi, C. Scala-Valero, Appl. Phys B, 66 (1998) 439.
[9] N. Pfeffer, T. Isoshima, M. Tian, T. Wada, J.M. Nunzi, H. Sasabe, Phys. Rev. A, 55 (1997) R2507; N. Pfeffer, Thesis, Université Paris-Nord (1994).