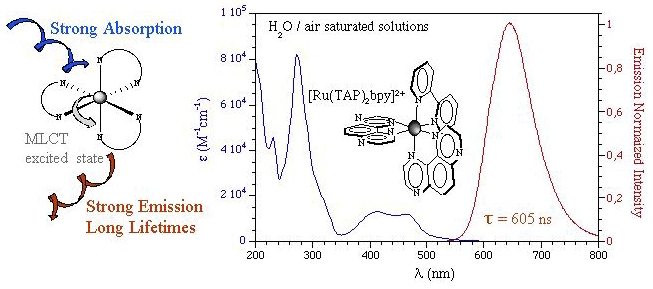
Photophysical Properties of Ru(II) Complexes

A. Masschelein, L. Jacquet, A. Kirsch-De Mesmaeker and J. Nasielski, Inorg. Chem., 1990, 29, 855-860
click on the absorption spectrum or the emission spectrum to go to the corresponding part or just scroll down the page
The choice of the ligands allows to tune the MLCT absorption. Indeed p-acceptors ligands will present a low lying p* orbital and in the same time stabilise the dp orbital centered on the metal by retro-coordination. This allows also to tune the redox properties as the lowest lying p* orbital of the ligands will be involved in the reduction processes and the dp orbital centered on the metal will be involved in the oxidation processes.
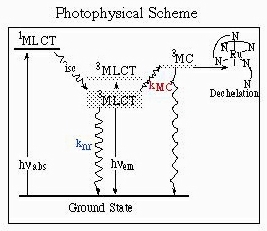
After inter-system crossing from the 1MLCT singlet excited state to the 3MLCT triplet state, several deactivations pathways can be followed :
In the Presence of DNA
The interaction of the complexes with DNA produce usually an increase of the emission intensity :
J.P. Lecomte, A. Kirsch-De Mesmaeker and G. Orellana, I. Phys. Chem., 1994, 98, 5382-5388
However, in some cases, when photreactions occurs between the complex and the DNA bases (photo-induced electron transfer), the luminescence is quenched in the presence of DNA as it is explain in the following part of the introduction "Photoreactions"
oxidation = abstraction of an electron from the HOMO = dp orbital centered on the metal.
reduction = addition of an electron on the LUMO = p* orbital centered on the ligand.
If the orbitals involved in the redox and in the spectroscopic processes (spectroelectochemical correlation) the redox potential in the excited state can be expressed as
Pred* = Pred + DE00
Pox* = Pox - DE00
where DE00 corresponds to the energy of the 0-0 transition from the 3MLCT excited state.
From these relation, one can see that the redox properties are easily tuned by the choice of the ligands considerring their p-acceptor strengths. This is of particular importance as one of our objective is to design photo-reactive complexes toward DNA (introduction page).