| Photoiupac home page | Discussion | Photobiology.com home |
Photoınduced energy-electron transfer studıes wıth naphthalene dıımıdes ın solutıon and ın polymer fılms
Sıddık İçli
a, Serap Alpb, Andrey O. Doroshenkoc, Canan Karapirea, Sule. Ertanb, andBanu Közb
1
Kimya Bölümü, Fen Fakültesi, Ege Üniversitesi, Bornova, 35100 İzmir2
Kimya Bölümü, Eğitim Fakültesi, Dokuz Eylül Üniversitesi, Buca, İzmir3
Institute for Chemistry at Kharkov State University, 310077 Kharkov UKRAINE
Abstract
Seven derivatives of naphthalene diimides, synthesized, have shown similar photophysical properties. Low fluorescence quantum yields(0.002-0.006) and short fluorescence lifetimes (5-18 ps) have suggested rapid intersystem crossing processes from excited singlet state. Quenching of fluorescence emissions of aromatic donor molecules, i.e. naphthalene, phenanthrene and pyrene, at rates reaching to diffusion limits in acetonitrile(2-8x10-10 M-1s-1), have proven the electron acceptor capacities of naphthalene diimides. Photooxydation of styrene to benzaldehyde, in presence of naphthalene diimide(NDI) molecule, is found to occur at similar rates respect to perylene diimides. Addition of ferric and cupric ions to NDI, have enhanced the formation rate of benzaldehyde about twofold. Photooxidation of a -terpinene with naphthalene diimide, produced only p-cymene, no endoperoxide adduct was identified. Naphthalene diimides appear to produce no singlet oxygen, and photooxidation probably occurs on radical chain reactions of super oxide anion radical. But the electron transfer electron via excited triplet or singlet state, remains to be unclear.
Key words ¾ ¾ N,N¢ -bis-aryl(alkyl) 1,4,5,8-naphthalenediimides, fluorescence quenching, photoinduced electron transfer, photooxydation.
Photophysical characterization of naphthalene diimides are limited to a few derivatives in literature. Politi et al. [ 1] reports the absorption and fluorescence parameters of N,N¢ -dibutyl derivative of naphthyl diimides. Fluorescence quantum yield is found to be very weak, Qf=0.002, due to the n-p * character of lowest excited states, such as in phthalimides. Whereas in 1,8-N-butylnaphthylimides lowest excited state has p -p * character, fluorescence quantum yield is in the range of Qf=0.01-0.20 in organic solvents and Qf=0.36 in water [ 2] . On the other hand naphthalene diimides are found to form stable anion radical on reduction [ 4] , as observed with other condensed aromatic diimides, i.e. perylene diimides [ 3] . Naphthalene diimides are expected to form anion radical intermediate on photo electron transfer processes, as detected in perylene diimides [ 5] .
We now report the photophysical characteristics of naphthyl diimides in solution, photoinduced energy transfer from aromatic p -electron donor molecules, and electron transfer from olefins on photooxidations.

Ar R .
I Phenyl V n-Butyl
II o-Chlorophenyl VI n-Dodecyl
III p-Tolyl VII Cyclohexyl
IV a -Naphthyl
2. Experimental details
2.1. Materials
1,4,5,8-Naphthalenedianhydride, aniline, o-chloroaniline, p-fluoroaniline, p-bromoaniline,
p-toluidine, cyclohexylamine, n-butylamine, n-dodecylamine, phenanthrene, pyrene and perylene were obtained from Fluka and Merck, and were used as supplied. All of the organic solvents that were used (acetonitrile, chloroform, methanol), were of spectrophotometric grade. a -Cyclodextrin was obtained from Avocado firm, and was used as supplied.
2.2. Organic synthesis
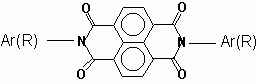
N,N¢ -bis-aryl(alkyl) 1,4,5,8-naphthalenediimides were prepared by the method that were employed for the synthesis of N-substituted perylene diimides [ 6] . 1,4,5,8-Naphthalenedianhydride was reacted with appropriate amine derivative in m-cresol/isoquinoline at elevated temperatures (> 200oC) for 4-6 hours in order to yield the naphthalenediimide. Molecular structures were analysed in IR(Fig. 1) and proton NMR spectra(Table 1 and Fig.2).
N,N¢ -bis-phenyl-1,4,5,8-naphthalenediimide, I: The mixture of 1,4,5,8-naphthalenedianhydride (1 g, 3.75 mmol) and aniline (0.7 g, 7.5 mmol) was dissolved in 20 ml m-cresol and a few drops of isoquinoline was added. The temperature was gradually increased to 200oC. The mixture was kept at this temperature for 24 hours. The viscous solution was diluted with 20 ml m-cresol and poured slowly into 30 ml of stirred acetone. The precipitate was filtered and washed thorughly with warm acetone. Crude product was re-dissolved in chlorofom and poured into acetone and filtered for purification. N,N¢ -bis-phenyl-1,4,5,8-naphthalenediimide, I, C24H14N2O4, M.W.: 396.4 g/mol, was obtained in 0.92 g, 62 % yield. Molecular structure was analysed in IR and proton NMR spectra as seen in Table 1.
N,N¢ -bis-n-butyl-1,4,5,8-naphthalenediimide, V: The mixture of 1,4,5,8-naphthalenedianhydride (1 g, 3.75 mmol) and n-butylamine (0.7 g, 10 mmol) was dissolved in 20 ml m-cresol and few drops of isoquinoline was added. The temperature was gradually increased to 160oC. The mixture was kept at this temperature for 6 hours under nitrogen. The viscous solution was diluted with 20 ml m-cresol and poured slowly into 50 ml of methanol while stirring. The precipitate was filtered and washed thorughly with warm acetone. Crude product was purified by column chromatography, using dichloromethane as eluent. N,N¢ -bis-n-butyl-1,4,5,8-naphthalenediimide, V, C22H22N2O4, M.W.: 378.4 g/mol, was obtained in 0.46 g, 32 % yield. Molecular structure was analysed in IR and proton NMR spectra(Table 1).
Table 1
: Characteristic absorptions of synthesized N,N¢ -bis-aryl(alkyl) 1,4,5,8-naphthalene-diimides at IR(n , cm-1, in KBr) and proton NMR Spectra (d , ppm in CDCl3/CF3COOD).Ar(R) n C=O n imide n C-O d Naph d N-Ar d N-CH d N-CH2 d Ar-CH3 d C-CH2 d C-CH3
I Phenyl 1711 1672 1124 7.26 7.34-7.62
(4H,s) (10H,m)
II o-Chlorph. 1718 1677 1057 8.94 7.38-7.42
(4H,s) (2H,dd)
7.47-7.57
(4H,m)
7.63-7.67
(2H,dd)
III p-Tolyl 1710 1670 1120 8.93 2.46
(4H,s) (6H,s)
IV a -Naphthyl 1714 1675 1120 8.91 7.45-7.47
(4H,s) (10H,m)
8.00-8.08
(4H,m)
V n-Butyl 1700 1650 1080 8.76 4.20 1.74(4H,m); 0.99 (4H,s) (4H,t) 1.46 (6H,t)
(4H,m)
VI n-Dodecyl 1707 1648 1080 8.72 4.16 1.71(4H,m); 0,85
1.33(4H,m);
1.22(4H,m)
VII Cyclohexyl 1710 1650 1100 8.68 4.99 2.46(4H,dq);
(4H,s) (2H,m) 1.87-1.91(4H,dbroad)
1.72-1.74(6H,dbroad);
1.23-1.48(6H,m)
2.3. Spectroscopic measurements
Synthesized compounds were analyzed at Schimadzu IR4570 spectrophotometer for IR spectroscopy, at JASCO V-530 UV-VIS spectrophotometer for UV-Visible spectroscopy, at JEOL JNM-GX 400FT 400 MHz NMR for NMR spectroscopy analysis. Fluorescence emission spectra were recorded at a PTI QM1 fluorecence spectrophotometer.
Fluorescence quantum yields of naphthalene diimides were measured in reference to absorption and fluoresence emission of anthracene, l exc= 357 nm.. GC-MS studies were performed with a Hewlett Packard HP 6890 instrument, using HP-5MS column at column temperature range of
40-200oC in 21 minutes.
3. Results and discussion
3.1. UV-VIS Spectroscopy Studies
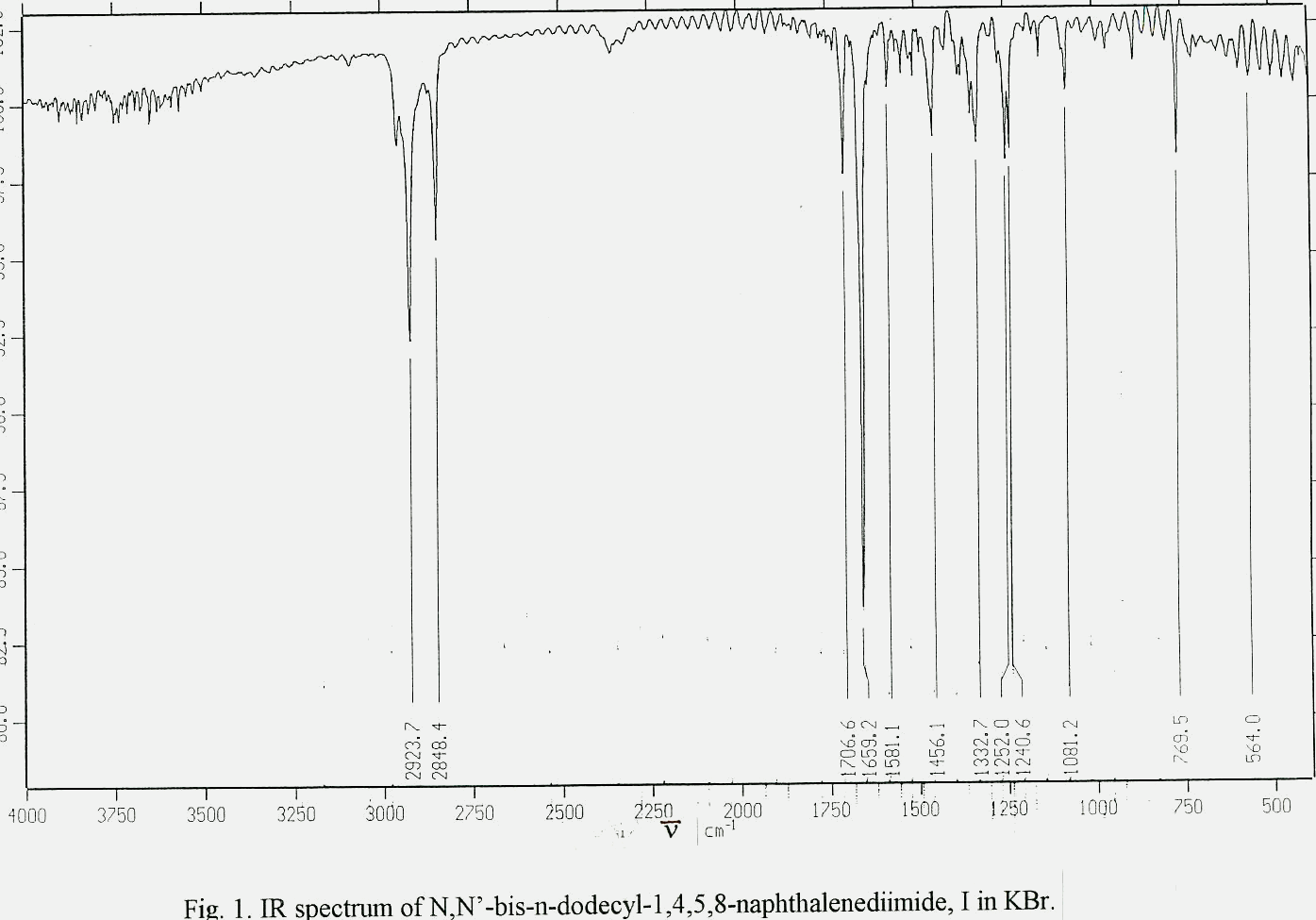
UV absorption spectra of naphthalene diimides have shown three charactheristic bands at 377 nm, 357 nm and 340 nm in acetonitrile solutions. In accordance with the reported data of Politi et al. [ 2] (Fig. 3 and Table 2). N-aryl(alkyl) substitution did not alter absorption wavelengths, l , and molar extinction coefficients, e . Solubility differences in acetonitrile were observed among the seven derivatives. Most soluble naphthalene diimides were found to be the alkyl derivatives of V, VI and VII. The n-butyl derivative, V, was observed to be the most soluble in acetonitrile, chloroform and dichloromethane solvents. The least soluble naphthalene derivative was o-chlorophenyl derivative, II. In general low solubilities of naphthalene diimides have caused the difficulties on measurements of absorption spectra. All the acetonitrile solutions were colorless.
A
l , nm
Fig. 3. UV absorption spectrum of N,N¢ -bis-phenyl-1,4,5,8-naphthalenediimide, I(PNDI), in
acetonitrile.
___________________________________________________________________________Table 2. UV-VIS Spectroscopic data (l , nm, and e , L.mol-1.cm-1) of synthesized N,N¢ -bis-aryl(alkyl) 1,4,5,8-naphthalenediimides in acetonitrile.
Ar(R) l 1 e 1 l 2 e 2 l 3 e 3 .
I
Phenyl 377 32500 357 27800 340 17160II
o-Chloroph. 376 42900 356 35100 339 20300III
p-Tolyl 376 32800 356 29500 339 18800IV
a -Naphthyl 377 33000 357 29000 340 17500V
n-Butyl 377 43500 356 35100 339 21100VI
n-Dodecyl 377 33900 357 28700 340 19100VII
Cyclohexyl 378 30600 358 25800 342 155003.2. Emission spectra and fluorescence quantum yields
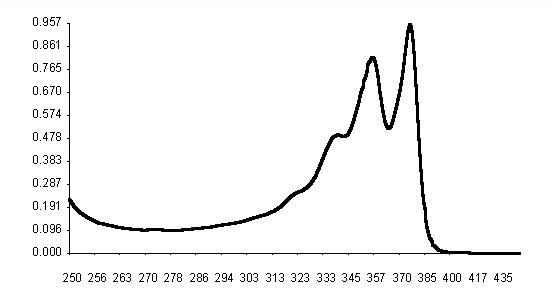
Figure 4 shows the absorption and emission spectra of N,N¢ -bis-n-butyl-1,4,5,8-naphthalenediimide, V, and N,N-bis-n-dodecyl-1,4,5,8-naphthalenediimide, VI, compounds.
Mirror image appareance of the absorption and emission spectra is evident. In general, the solvent variation does not alter the absorbtion and emission spectra, indication of very limited vibrations and evidence to the very rigid structure of the naphthalene diimide molecule.
The radiative lifetimes, t o, were calculated by the formula: t o = 3.5x108/n 2max.e max. D n 1/2 , where n max is the wavenumber in cm-1, e max is the molar extinction coefficient at the selected absorption wavelength, and D n 1/2 is the half width of the selected absorption in wavenumber units of cm-1[ 7] . Fluorescence lifetimes are estimated as t f = t o.Qf and the rates of fluorescence as kf = 1/t o (Table 3).
A
l , nm
Fig. 4. Absorption (Ab) and emission(Em, l exc= 356 nm) spectra of N,N¢ -bis-n-butyl-1,4,5,8-
naphthalenediimide, V, in acetonitrile.
Fluorescence quantum yields are observed to be in the range of 0.002-0.006 in acetonitrile. A rapid intersystem crossing probably quenches the singlet excited state of naphthalene diimides [ 8] . Calculated fluorescence lifetimes are seen to be in the range of 5-18 ps, another evidence of ultra fast intersystem crossing from singlet excited state. This value is in good correlation with the literature data, Fox [ 9] reports fluorescence lifetimes for naphthalene diimides at lower values than 20ps. ___________________________________________________________________________
Table 3. Fluorescence quantum yields,Qf, radiative lifetimes, t o(ns), fluorescence lifetimes, t f(ps), fluorescence rate constants, kf(108 s-1), and singlet energies, Es(kcal/mol) data of naphthalene diimides in acetonitrile.
Ar (R) l max e max Qf t o t f kf Es
I
Phenyl 377 32500 0.002 3.1 6.2 3.2 75.9II
o-Chloroph. 376 42900 0.002 2.3 4.6 4.3 76.1III
p-Tolyl 376 32800 0.004 3.1 12.4 3.2 76.1IV a
-Naphthyl 377 33000 0.005 3.1 15.5 3.2 75.9V
n-Butyl 377 43500 0.006 2.3 13.8 4.3 75.9VI
n-Dodecyl 377 33900 0.006 3.0 18.0 3.3 75.9VII
Cyclohexyl 378 30600 0.004 3.1 12.4 3.2 75.7Politi et al. reports that 1,4,5,8-naphthalene diimides are soluble in a -cyclodextrin(a -CD) via complex formation[ 8] . Absorption and emission spectra of n-butyl derivative of naphthalene diimide is being demonstrated in water solutions. N,N¢ -bis-n-butyl-1,4,5,8-naphthalenediimide, V, was stirred in 0.05 M aqueous solution of a -cyclodextrin for 6 hours, warmed for 0.5 hour and cooled solution was filtered from 0.2 m m pore size Acrodisc filters. Absorption spectrum of V had shown the bands at 387 nm, 365 nm and 348 nm in a -CD aqueous solution, same as reported values. The emission spectrum, obtained at excitation of 345 nm, has yielded a quantum value, Qf, of 0.031, that is fivefold higher respect to Qf of V in acetonitrile solution(Table 3). Literature reports the increase of Qf of n-butyl derivative from 0.001 in acetonitrile to 0.014 in a -CD aqueous solution[ 8] . But all the attempts to dissolve all other six naphthalene derivatives in a -cyclodextrin have failed, in spite of warming and stirring for several days. No absorption spectra were detected from filtrates. It may be that only the n-butyl aliphatic chain fits into the cyclodextrin cone, as proposed by Politi[ 8] .
3.3. Fluorescence quenching studies
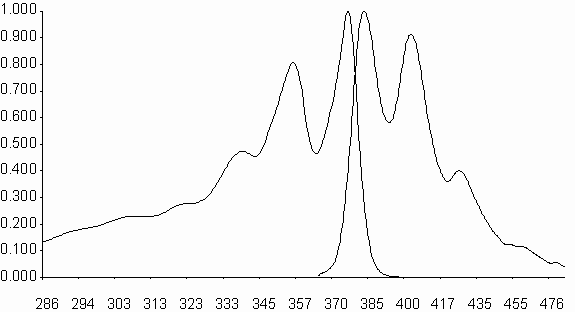
Naphthalene diimides are known to be electron acceptors [ 9] . N,N¢ -bis-aryl(alkyl)-1,4,5,8-naphthalenediimides are found to quench effectively the fluorescence emissions of aromatic donor molecules of naphthalene, phenanthrene, pyrene and perylene, in spite of their very low fluorescence quantum yields. Figure 5 shows the quenching of fluorescence emissions of perylene by addition of N,N¢ -bis-N-phenyl-1,4,5,8-naphthalenediimide, I,(PNDI) and the Stern-Volmer plot. All the spectra were corrected for inner and absorption effects before calculations. Calculated quenching rates are shown in Table 4. It is seen that quenching rates are about on the diffusion limits for all the aromatic hydrocarbons. The differences of kq values are seen to be about threefold from naphthalene to phenanthrene (from 2.6x1010 to 7.2x1010 M-1s-1) and fourfold from pyrene to perylene(from 1.9x1010 to 7.6x1010 M-1s-1). These enhancements may be attributed to an increase on aromaticity of the condensed ring systems. A similar study with perylene diimides has resulted in enhancement of quenching rates in paralel to an increase in aromaticity of condensed rings [ 10] . But the rates of quenching were reported to have increased fivefold from naphthalene to phenanthrene (from 4.4x1011 to 2.8x1012 M-1s-1) and hundredfold from phenanthrene to dihydrocarbazolo-carbazole (from 2.8x1012 to 2.5x1014 M-1s-1) in presence of perylene diimides. Energy transfer is seen to be more effective between aromatic donors and perylene diimides, respect to between aromatic donors and naphthalene diimides. In general, fluorescence quenching of aromatic donors by naphthalene diimides and perylene diimides may assumed to be comparative scale.
Table 4. Fluorescence quenching rate consttants of condenssed aromatic p -electron donor molecules by the addition of N,N¢ -bis-N-phenyl-1,4,5,8-naphthalendiimide, I,(PNDI) in acetonitrile.
Compound_________ kq(M-1.s-1)_________
Naphthalene (2.63± 0.55)x1010
Phenanthrene (7.18± 1.36)x1010
Pyrene (1.91± 0.24)x1010
Perylene (7.62± 0.65)x1010
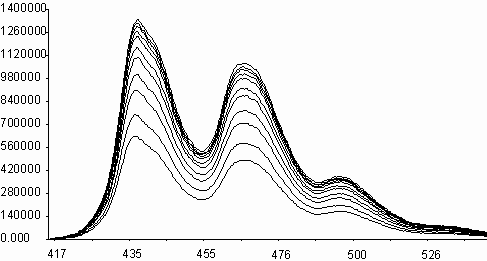
l ,nm
I0/I
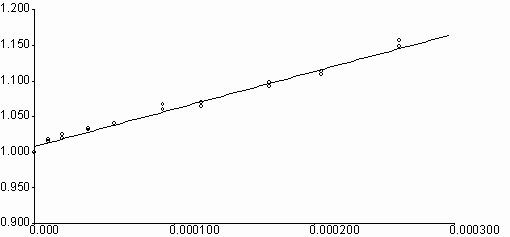
[ C]
Fig. 5. Fluorescence quenching of perylene(l exc = nm) by N,N¢ -bis-phenyl-1,4,5,8-
naphthalenediimide, I(PNDI), in acetonitrile at quencher concentrations of
1x10-5 - 3x10-4 M (A) and the Stern-Volmer plot (B).
3.4. Photooxidations with naphthalenediimides
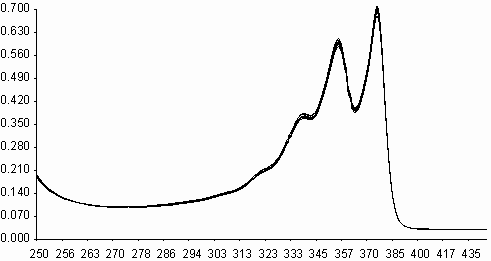
Similarities on molecular structures and on some photophysical properties between naphthalene diimides and perylene diimides, may suggest a similarity on photooxidations also[ 5,11] . Fluorescence quenching rates of aromatic hydrocarbons of naphthalene diimides, exceeding the diffusion limits(kq> 1010 M-1s-1), may point that an electron transfer may occur on irradiation leading to photochemical reactions. Photooxidation of styrene to benzaldehyde in acetonitrile solution was studied in presence of N,N¢ -bis-N-phenyl-1,4,5,8-naphthalenediimide, I (PNDI). The formation of benzaldehyde was monitured in UV spectra. As seen in figure 6, PNDI is photostable under irradiation of 377 nm for 80 minutes. The UV spectra of PNDI with styrene in absence and in presence of ferric ions (ferric myristate) or cupric ions (cupric pivalate) during irradiation times of 0-75 minutes are given in figures 7 and 8, respectively. Perylene diimides are known to photooxidize olefins through singlet electron transfer yielding super oxide [ 5] . In order to compare, styrene was
oxidized in acetonitrile solution with N,N¢ -bis-N-dehydroabietyl-3,4,9,10-perylenediimide, (PERIM) under the same conditions. The results are given in Table 5. It is seen that PNDI oxidizes styrene in 0.40 % to benzaldehyde, where the same ratio is as 0.52 % with PERIM, in absence of metal ions. In presence of ferric and cupric ions, photooxidation is enhanced both with PNDI and PERIM about twofold.
A
 l
,nm
l
,nm
Fig. 6. UV absorption spectra of N,N¢ -bis-phenyl-1,4,5,8-naphthalenediimide, I(PNDI), in
acetonitril solution during irradiation under 400 W sodium lamp for 0-80 minutes
i) Irradiation of styrene solution at presence of PNDI
A
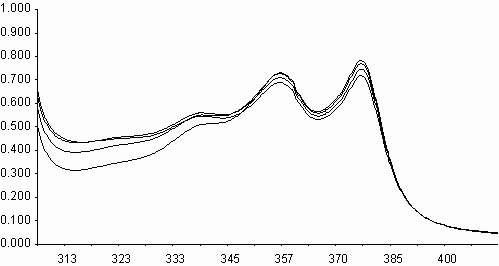
l ,nm
A
ii) Irradiation of styrene solution at presence of PNDI and Fe+3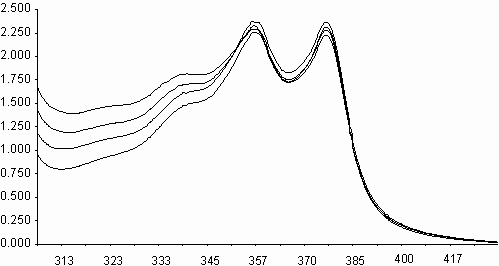 l
,nm
l
,nm
Fig. 7. UV absorption spectra of N,N¢ -bis-phenyl-1,4,5,8-naphthalenediimide, I(PNDI), in
acetonitrile, during irradiation under 400 W sodium lamp for 0-75 minutes i) in
presence of styrene, and ii) in presence of styrene and ferric myristate.
A
Irradiation of styrene solutionat presence of PNDI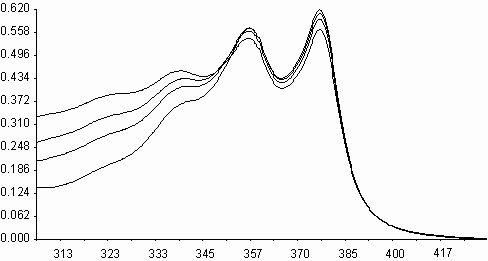
l ,nm
A
Irradiation of styrene solution at presence of PNDI and Cu+2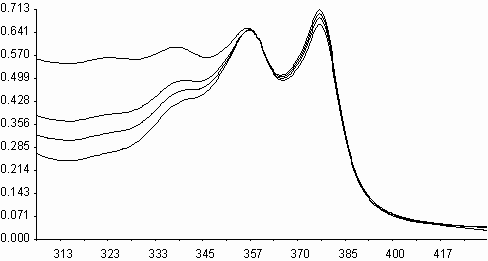 l
,nm
l
,nm
Fig. 8. UV absorption spectra of N,N¢ -bis-phenyl-1,4,5,8-naphthalenediimide, I(PNDI), in
acetonitrile, during irradiation under 400 W sodium lamp for 0-75 minutes i) in
presence of styrene, and ii) in presence of styrene and cupric pivalate.
Table 5. Photooxydation of styrene in presence N,N-bis-N-phenyl-1,4,5,8-naphthalenedi-imide, I,(PNDI) or N,N-bis-N-dehydroabietyl-3,4,9,10-perylenediimide, (PERIM) with and without ferric(Fe+3) and cupric(Cu+2) ions after 75 minutes irradiation.
Sensitizer PNDI PNDI PERIM PERIM
Sensitizer concentrations 2.3x10-5 M 7.3x10-5 M 1.9x10-5 M 0.6x10-5 M
Fe+3 ---- Present ------- Present
Initial styrene concentration 1.730 M 1.657 M 1.717 M 1.733 M
Benzaldehyde conc. 0.0062 M 0.0174 M 0.0089 M 0.0106 M
Benzaldehyde yield 0.36 % 1.05 % 0.52 % 0.61 %
Sensitizer PNDI PNDI
Sensitizer concentrations 2.3x10-5 M 7.3x10-5 M
C u+2 ---- Present
Initial styrene concentration 1.727 M 1.727 M
Benzaldehyde conc. 0.069 M 0.0110 M
Benzaldehyde yield 0.40 % 0.64 %
___________________________________________________________________________
These results may suggest that the formation of super oxide from naphthalene diimide anion radical on photooxidation may be a logical route, as found in perylene diimides [ 5,11] . Formation of stable naphthalene diimide anion radical is proven at electrochemical reduction studies[ 12,13] . Fox reports an effective intramolecular photoinduced electron transfer from nitroxyl radicals to 1,4,5,8-naphthalene diimide moiety[ 9] . In agreement with, Wasielewski has detected ultrafast photoinduced electron transfer in the triad molecule of zinc methyl 131-desoxopyropheophor-bide a-pyromellitide-1,4,5,8-naphthalenediimide[ 14] . It is expected that excited naphthalene diimide, PNDI, molecule would abstract an electron from olefinic bonds to form a naphthalene diimide anion radical, that would transfer the electron to oxygen in an exciplex to cause oxydation reactions. The presence of metal ions of ferric and cupric, most likely promotes the formation of super oxide anion radical, via electron transfer from metal ion to excited naphthalene diimide in an exciplex to form the naphthalene diimide anion radical, and may result in enhanced rates of oxidation. The photoinduced rapid energy transfer from singlet to triplet S1® Tn, suggests that an electron transfer would occur from triplet state in naphthalene diimides, in contrast to singlet excited state energy-electron transfer for perylene diimides. On the other hand Fox reports an energy gap of 27 kcal/mol for S1-T1 for n-dodecyl derivative of naphthalene diimide. Perylene diimides have the same S1-T1 energy gap. It seems that photo oxidative ability of naphthalene diimides are similar to perylene diimides.
a -Terpinene is known to produce endoperoxide adduct (ascardiol) on photooxidation with triplet sensitizers (rose bengal, tetraphenylporphrine-TPP).

 It is also proven that a
-terpinene is being converted to p-cymene in presence of perylene diimide[
5]
under solar irradiation, through a singlet state electron transfer in an exciplex, that forms hydroperoxy radicals reaching to p-cymene in radical chain reactions. a
-Terpinene (0.1 M) and few mg of N,N¢
-bis-N-(n-butyl)-1,4,5,8-naphthalenediimide, V(BNDI) in 100 ml acetonitrile, was irradiated with a 400 W sodium lamp for 15 hours. Analysis in GC-MS has revealed the formation of a single product, identified as p-cymene (most abundant peaks in MS, m/e: 134; M.P., 119(B.P.); MePhCHCH2, 91; Ph-CH2, 77; Ph).
It is also proven that a
-terpinene is being converted to p-cymene in presence of perylene diimide[
5]
under solar irradiation, through a singlet state electron transfer in an exciplex, that forms hydroperoxy radicals reaching to p-cymene in radical chain reactions. a
-Terpinene (0.1 M) and few mg of N,N¢
-bis-N-(n-butyl)-1,4,5,8-naphthalenediimide, V(BNDI) in 100 ml acetonitrile, was irradiated with a 400 W sodium lamp for 15 hours. Analysis in GC-MS has revealed the formation of a single product, identified as p-cymene (most abundant peaks in MS, m/e: 134; M.P., 119(B.P.); MePhCHCH2, 91; Ph-CH2, 77; Ph).
These results prove that naphthalene diimides do not form singlet oxygen as the other triplet sensitizers. Electron transfer, from singlet or triplet level would produce the super oxide anion radical, which may cause the formation of hydroperoxyl radicals. Radical chain reactions of hydroperoxyl radicals would result in formation of benzaldehyde from styrene and p-cymene from a -terpinene. However, the mechanism of photoinduced energy-electron transfer with naphthalene diimides appear to be still unclear under the light of present data.
Conclusions
We have demonstrated that N-substituted naphthalene diimides have very short fluorescence lifetimes (5-18 ps) and quench the fluorescence emissions of electron donors above diffusion limits (2-8x10-10 M-1s-1). It is detected that the solubility in a -cyclodextrin aqueous solution is only limited to n-butyl derivative, in contrast to generalization in literature. Photooxidation studies have proven that naphthalene diimides exert electron transfer via singlet or triplet excited state, no evidence to singlet oxygen reaction was observed. An ultrafast energy transfer from singlet excited state is evident, but the electron transfer from singlet or from triplet state is unclear.
Acknowledgements
We thank to Professor Hans Gerhard Loehmannschroeben for providing some of the used chemicals, and some NMR , IR spectra. We acknowledge the project support funds of Research Center of Ege University (EBILTEM), to State Planning Organization of Turkey (DPT), to Scientific Research Council of Turkey (TUBITAK) and to Alexander von Humboldt Foundation of Germany
References
[ 1] T.C. Barros, S. Brochsztain, V.G. Toscano, P.B. Filho and M.J. Politi, J.
Photochem. Photobiol. A: Chem. 111 (1997) 97.
[ 2] T.C. Barros, G.R. Molinari, P.B. Filho, V.G. Toscano and M.J. Politi, J.
Photochem. Photobiol. A: Chem. 76 (1993) 55.
[ 3] H. Langhals, Heterocycles, 40(1) (1995) 477.
[ 4] L.L. Miller, C-J. Zhuang and P. Kasai, Jour. Am. Chem. Soc. 115 (1993) 5982.
[ 5] L.Chen, L.A. Lucia, E.R. Gaillard, H. Icil, S. Icli and D.G. Whitten, Jour. Phys.
Chem. 102 (1998) 9095.
[ 6] H. Icil and S. Icli, J. Polym. Sci. A: Poly. Chem. 35 (1997) 2137.
[ 7] N.J. Turro, Molecular Photochemistry, W.A. Benjamin Inc.,London, pp 44-64, 1965.
[ 8] S. Brochsztain and M. J. Politi, Langmuir 15 (1999) 4486.
[ 9] S. Green and A. Fox, J. Phys. Chem. 99 (1995) 14752.
[ 10] S. Icli, H. Icil and I. Gurol, Tr. J. Chem. 21 (1997) 363.
[ 11] S. İçli, Ş. Demiç B. Dindar, A.O. Doroshenko and C. Timur, submitted to J. Photochem.
Photobiol: A Chem..
[ 12] J-F. Penneau, B. J. Stallman, P. H. Kasai and L. L. Miller, Chem. Mater. 3 (1991) 791.
[ 13] G. Heywang, L. Born, H-G. Fitzky, T. Hassel, J. Hocker, H-K. Müller, B. Pittel and S.
Roth, Angew. Chem. Int. Ed. Engl. 28 (1989) 483.
[ 14] G. P. Wiederrecht, M. P. Niemczyk, W. A. Svec and M. R. Wasielewski, J. Am. Chem.
Soc. 118 (1996) 81.