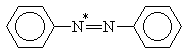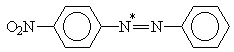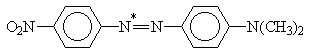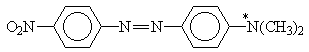PHOTOINDUCED PROTONATION OF DYES IN POLYMER MATRIX.
NOVEL EFFECTIVE MODE FOR CONVERSION AND STORAGE OF SOLAR ENERGY. THEORETICAL APPROACH FOR
PREDICTING THE LIGHT ENERGY STORAGE EFFICIENCY
Ivan Petkov* , Todor Dudev, Nadejda Sertova,
Todor Deligeorgiev and Veneta Dryanska
University of Sofia, Faculty of Chemistry, 1 James Bourchier Avenue,
1126 Sofia, Bulgaria
ABSTRACT
The possibilities of polymer (polyvinyl chloride, PVC) films, doped with two kind of dyes are discussed as a novel type of POLYMER LIGHT ENERGY STORAGE SYSTEM (PLESS). On the basis of quantum mechanical calculations is made attempt in the prediction of the energy storage efficiencies of some 3-aryl-2-(2-benzothiazolyl)-acrylonitriles and azo dyes during the process of the photoinduced protonation of the dyes into the polymer matrix. In PLESS the energy of the incident light is transformed and accumulated as a photoinduced chemical change of the storage system. The process comprises two steps: photochemical degradation of the polymer and the receiving of HCl and protonation of the dyes. The results show that the efficiency of the energy accumulation of the process of protonation is very high -about 80-90%. The advantages of employing photoinduced protonation become clear in comparing these values for the process of trans-cis isomerization of azobenzene which was calculated to be 1.6%. Obviously, the influence of various other factors have to be taken into account in experimental conditions. The simplicity of the system proposed, the large number of appropriate dyes and polymer materials available, the high energy storage efficiencies found, and the possibility of asymmetric membrane formation make the PLESS a promising candidate for solar energy conversion and storage.
Key words: Photoinduced protonation, solar energy storage, energy storage efficiency, dyes, polymer matrix.
*Correspondence to: Dr I. Petkov, Present address – Shizuoka University, Research Institute of Electronics, 3-5-1 Johoku, Hamamatsu, 432-8011, Japan.
Tel. +81-53-478-1332
Fax: +81-53-478-1348
E-mail: roivan@ipch.shizuoka.ac.jp
INTRODUCTION
The photochemical conversion and storage of solar energy have been an object of many investigations performed over the last decades: the solar energy is the most important and practically unlimited source of regenerative energy. It has been understood that development and implementation of cheap and functional systems for efficient solar energy conversion and storage is of crucial importance for the humankind's future.
Two main factors have to be considered in assessing the potential of newly developed systems for solar energy storage, namely: (1) the storage capability, and (2) the price to be paid. Presently available on the market solar cell devises are based mainly on silicon. They exhibit conversion efficiencies of about 10-15%. Technological process of fabrication is, however, rather sophisticated and expensive.
Devices based on organic materials have some advantages and can successfully compete in this area. They are quite attractive with the lower prices and easy fabrication. To be applied in practice, however, they must possess special characteristics - combination of suitable physical, chemical, electrophysical, spectral, photochemical, mechanical and other properties. It should be noted that at this stage their photochemical properties are far behind those of their inorganic competitors. This is the reason that the number of investigations in this field is quite significant and constantly growing.
In the present paper we focus our attention on the photochemical processes taking place in dye-doped polymer materials. The process of photoinduced protonation of dye molecules in the polymer matrix is considered. The applicability of the system proposed for practical use is discussed.
RESULTS AND DISCUSSION
In the polymer light energy storage system (PLESS) the energy of the incident light is transformed and accumulated as a photoinduced chemical change of the storage system. The process comprises the following steps:
Step one.
The sensitizer (usually PVC) absorbs photons (photodegradation process) and releases HCl:
polymer ![]() polymer* + HCl
(1)
polymer* + HCl
(1)
The asterisk signifies that the respective molecule is in electronically excited state.
Step two.
Dye molecule which is trapped into the polymer matrix interacts with HCl. The necessary condition is that the molecule possesses basic centre(s). This process may involve dye molecule in its ground electronic state:
dye + HCl ![]() [dyeH+] Cl
[dyeH+] Cl
dye + HCl ![]() [dyeH+] Cl
[dyeH+] Cl
dye + HCl ![]() [dyeH+] Cl- (2)
[dyeH+] Cl- (2)
Dye molecules may be excited by the incident light and the protonation may also occur in their electronically excited state. Usually excitation to S1 state is considered. The protonation in this case is a multistep process:
dye ![]() dye*
dye* ![]() ([dyeH+] Cl
([dyeH+] Cl
dye ![]() dye*
dye* ![]() ([dyeH+] Cl
([dyeH+] Cl
dye ![]() dye*
dye* ![]() ([dyeH+] Cl
([dyeH+] Cl
dye ![]() dye*
dye* ![]() ([dyeH+] Cl-)*
([dyeH+] Cl-)* ![]() [dyeH+] Cl- (3)
[dyeH+] Cl- (3)
The respective protonated dye forms which are metastable and energy-rich species are finally obtained. Thus, part of the energy of the incident light is converted and accumulated as an energy of the storage system. This can be utilized either as a heat or as an electrical current. The overall storage efficiency h w for each couple dye/[dyeH+]Cl_ will depend on the energy difference between the two forms, the respective energy barrier, the life-time of the protonated form, the presence of competing processes, etc. It has been shown [1] that h w can be regarded as a product of four efficiency factors:
h w = h E f h a h abs (4)
In Eq. (4) h E, the energy storage efficiency factor, is the ratio between the accumulated molar enthalpy D H of the process
dye + H+ ![]() dyeH+ (5)
dyeH+ (5)
and the energy of 1 mole of photons with a threshold wavelength l g [2].
![]()
![]()
![]() (6)
(6)
where h is the Planck constant, c - velocity of light and NA - Avogadro number, f is the quantum yield of the photochemical process, h a is the ratio between the energy of one mole of photons with the threshold wavelength l g and that of the total incident solar energy, and h abs is the ratio between the number of absorbed by the system photons and that of the total incident photons.
The energy storage efficiency factor h E for a given system can be evaluated theoretically. Thus, the potential of the system for an energy storage can be assessed prior to synthesized the respective substances, which sometimes are complex and quite expensive. h E can be calculated without much difficulty by quantum mechanical calculations. In the present paper, by employing quantum mechanical calculations, we obtained h E factors for some representatives of two classes of dyes:
3-aryl-2-(2-benzothiazolyl)-acrylonitriles
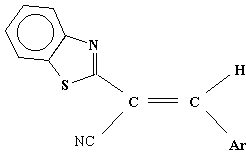
Ar = C6H5, 4-NO2C6H4,
4-(CH3)2NC6H4, C6H5CH=CH-,
and azo dyes
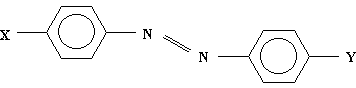
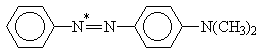
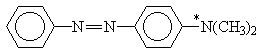
X = H, NO2; Y = H, N(CH3)2
Calculations were performed at AM1 (Austin Model 1) semi-empirical level [3] by employing Gaussian 92W program package [4]. Monoprotonated forms were considered only. The results obtained for the two groups of molecules are given in Tables 1 and 2.
Table 1. AM1 calculated energy storage efficiency factors for the process of protonation of some derivatives of 3-aryl-2-(2-benzothiazolyl)-acrylonitrile
Substituent |
h E a (%) |
C6H5 |
80.7 |
4-NO2C6H4 |
87.3 |
4-(CH3)2NC6H4 |
83.7 |
91.9b |
|
C6H5CH=CH- |
85.6 |
|
88.7 |
aThe value refer to protonation at the nitrogen atom in the benzothiazolyl ring unless indicated.
bThe value refer to protonation at the nitrogen atom in the dimethylamino group.
Table 2. AM1 calculated energy storage efficiency factors for the process of protonation of some azo dyesa
Molecule |
h E (%) |
|
|
82.5 |
|
|
91.2 |
|
|
82.8 |
|
|
86.6 |
|
|
94.8 |
|
|
97.3 |
|
aThe sites of protonation are marked by asterisks.
For molecules possessing several N-atoms, different h E values are predicted depending on the structure of the respective protonated forms. The results summarized in Tables 1 and 2 show that the efficiency of energy accumulation of the process of protonation is very high - about 80-90 percent. The advantages of employing photoinduced protonation become clear in comparing these values with h E for the process of trans-cis isomerization of azobenzene which was calculated to be 1.6%. It should be kept in mind that h E is one of the four quantities determining the overall storage efficiency of the photochemical process [Eq. (4)]. Obviously, the influence of various other factors have to be taken into account in experimental conditions.
As mentioned, the protonation process may take place in either ground or excited state of dye molecule. Which species will preferably be protonated depends on the relative basicity of the N-atoms in the respective species. Usually, in excited state nitrogen atoms become more basic [5] and, respectively, more attractive for proton attack. Thus, if appropriate l g of illuminating beam is chosen, the light energy can be utilized in two ways: (1) HCl releasing from the polymer matrix, and (2) exciting of dye molecule to S1 state where the protonation process is more efficient. It should be noted here that PVC material produces HCl under irradiation with UV, visible or infrared light. The annual energy input of solar irradiation on Earth is 5% UV, 43% visible and 52% infrared. This means that the PLESS can very effectively use the whole spectrum of solar irradiation.
CONCLUSIONS
The new polymer light energy storage system (PLESS), in our opinion, appears quite promising for developing light energy storage devices due to the following reasons:
(1) It is simple and inexpensive;
(2) The process of photoinduced protonation is characterized with high energy storage efficiencies;
(3) The number of dyes possessing basic centres is very large;
(4) There are various host systems donating protons under light illumination which can be PVC, polymers doped with protons releasing compounds, or special polymers with chemically implanted dyes and proton donating acids;
(5) If an appropriate device is employed, the process of photoinduced protonation can result in formation of asymmetric membranes with well separated charges. Thus, the light energy can directly be converted into electric current [6].
In a series of succeeding articles we will give more details about light energy storage capacity of different classes of dyes in polymer matrices. The photoelectrochemical behaviour of some asymmetric membranes formed will also be considered.
REFERENCES
J. R. Bolton. Solar Power and Fuels. New York, Academic Press, USA, 1977.
C. Philippopoulos and K. Marangozis. Kinetics and Efficiency of Solar Energy Storage in the Photochemical izomerization of norbornadiene to quadricyclane. J.Ind. Eng. Chem. Prod. Res. Dev. 23, 458, 1984.
M. J. S. Dewar, E. G. Zoebisch, E. F. Healy and J. J. P. Stewart. AM1: A new general purpose quantum mechanical molecular model. J.Am.Chem.Soc., 107, 3902, 1985.
M. J. Frisch, G. W. Trucks, M. Head-Gordon, P. M. W. Gill, M. W. Wong, J. B. Foresman, B. G. Johnson, H. B. Schlegel, M. A. Robb, E. S. Replogle, R. Gomperts, J. L. Andres, K. Raghavachari, J. S. Binkley, C. Gonzales, R. L. Martin, D. J. Fox, D. J. Defrees, J. Baker, J. J. P. Stewart and J. A. Pople, "Gaussian 92, Revision E.1", Gaussian, Inc., Pittsburgh PA, 1992.
S. G. Sculman. Modern Fluorescence Spectroscopy., (Editor E.L. Wehry), Vol. 2, Plenum Press, New York, pp 239-275, 1976.
J-I. Anzai and T. Osa. Photosensitive artificial membranes based on azobenzene and spirobenzopyran derivatives. Tetrahedron 50, 4039, 1994.