PREVITAMIN D PHOTOSYNTHESIS IN VITRO
Olga Dmitrenko
1 and Wolfgang Reischl21
Institute of Surface Chemistry, Kiev, Ukraine (odmitr@udel.edu)2
Department of Organic Chemistry, University of Vienna, A-1090 Vienna, AustriaI. Introduction
II. Media-induced effects
III. Effects of UV-irradiation
IV. Theoretical studies and molecular modeling
V. Summary
VI. References
I. Introduction
The UV part of sunlight which penetrates the earth┤s atmosphere can cause beneficial and detrimental effects on living organisms [1]. The vitally important vitamin D synthesis is induced by natural UV-irradiation in the epidermis [2]. This reaction sequence involves the photochemical ring-opening of the steroidal precursor 7-dehydrocholesterol (provitamin D) to previtamin D and subsequent thermal rearrangement to the prohormone vitamin D [3]. It can be viewed as a "relict from primeval times when many organic substances which are essential for the generation of living material, were formed under the influence of radiation energy through a combination of photochemical and thermal reactions" (Havinga, 14th Paul Karrer Lecture, presented 1973 in the Aula of the University of Zurich, [3]).
In contrast to the highly specific photoreaction occurring in skin [4a], photosynthesis of previtamin D in solutions (Figure 1) is complicated by a number of side-reactions, among which the undesired cis-trans isomerization of provitamin D (Pro) into tachysterol (T) is most effective. Besides of this cis-trans isomerization, photochemical formation of the so-called over-irradiation products (toxisterols, Tox) takes place [4,5].
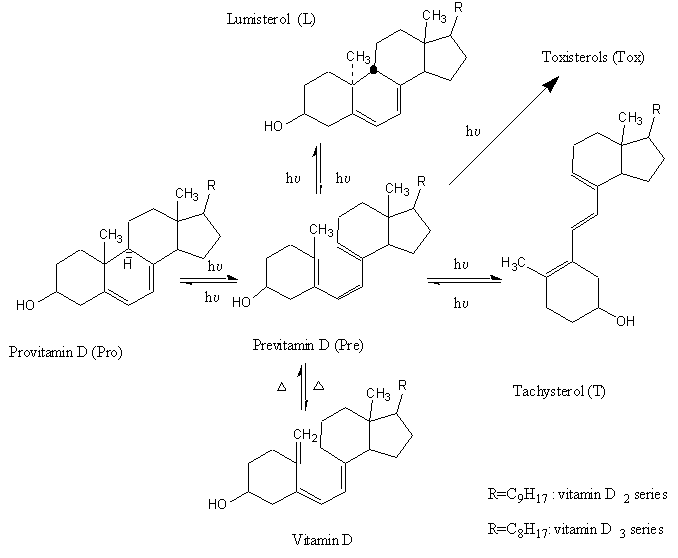
Figure 1. Scheme of provitamin D photoisomerization
Numerous studies on previtamin D photoconversions in various heterogeneous media as models of the epidermal environment [4] have been undertaken, but it is still unclear how the human body is able to use very small portion of the solar ultraviolet light. At the earth┤s surface the sunlight contains only UV-light of wavelengths larger than 280 nm which penetrates only the very upper layers of the skin [2d]). Still the transformation of provitamin D to previtamin D is highly efficient. In this connection, it should be mentioned that a series of weak absorption bands of provitamin D which occur around 330 nm (the main intensive absorption band is lying in the range from 250-300 nm) may account for epidermal photosynthesis, and the mechanism of the ring opening may possibly be different from that occurring at shorter wavelengths [4a)]. Additionally, effects which may be responsible for specificity of this reaction in vivo are specific and non-specific interactions with the surrounding environment [4g] (medium-induced effects) that may affect both the excited and ground states of the reactants; the decrease of conformational space available for the flexible triene molecules; the change in mobility of reactants and possible removing of the products from the photoreaction zone by circulation [4h]; and the presence of other UVB-photoactive centers which may transfer the energy of photoexcitation.
This list can be extended. Here we will mainly focus on the physical external effects: induced by reaction media and irradiation. Different theoretical models for the previtamin D photosynthesis will be discussed as well.
II. Medium-induced effects
The reaction of previtamin D photosynthesis is highly sensitive to its microenvironment. For instance, the amount of toxisterols after prolonged irradiation of provitamin D or previtamin D is influenced by the solvent [5, 6]. A conformational origin was shown to be the main reason for different toxisterols formation [6]. The reversible photoreactions emanating from previtamin D are dependent on the population of distinct conformers of previtamin D. Based on the principle of Non-Equilibration of Excited Rotamers (NEER) [5a], and considering the very short lifetime of the first excited singlet state, one may expect that the ratio of primary previtamin D photoproducs reflect the initial equilibrium composition of the ground state conformations. In this picture, the cZc conformation of previtamin D would be a precursor of the ring-closed products provitamin D (Pro) and lumisterol (L), while the tZc conformation leads predominantly to the trans-isomer tachysterol (T) (Figure 2) [7].
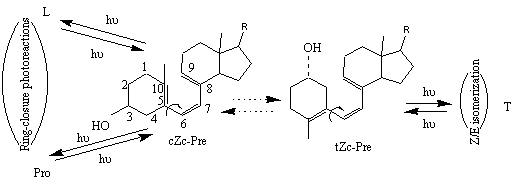
Figure 2. Simplified two-conformation scheme of previtamin D reversible photoconversions. Here and below, Z denotes cis geometry in relation to the C6-C7 double bond. Letters c and t refer to the s-cis and s-trans conformations of the C5-C6 and C7-C8 single bonds.
Conformational equilibrium of previtamin D is governed by a complex set of intramolecular interactions and represent a very sensitive system to explore changes of external factors [8]. In this view, the effect of solvent polarity on the reaction of provitamin D photoisomerization has been considered as the result of a balance between intramolecular and intermolecular electrostatic and steric interactions favoring the cZc conformation [9]. Another factor, which may change significantly the ground state previtamin D conformational equilibrium is the use of media which restrict the conformational flexibility and/or result in the preference of a certain geometry. This can be solvent matrices at low temperatures [10] or various heterogeneous molecular systems.
Synthetic lipid bilayers and multibilayers are the first potential candidates for mimicking the ordered milieu of the epidermis. The photoisomerization of provitamin D in phospholipids and liposomes have been studied in some details and compared with the reaction in homogeneous hexane solution [4a,b]. Photolysis of provitamin D within an ordered matrix results in decreased formation of tachysterol. Accumulation of lumisterol was found to be of the same order as in hexane solution [4a,b]. A conformational origin of this effect caused by geometrical restriction of the lipid has been suggested. Being formed in such an environment, previtamin D still assumes the shape of its precursor provitamin D, thereby fixing the cZc conformation. Under such geometric restriction a Z/E isomerization into tachysterol seems not very likely according NEER principle [5a,7]. Recently, Holick and Tian published their structural model for the position of cZc-previtamin D in membrane phospholipids. According to their picture previtamin D is aligned parallel to the phospholipids, with its 3-hydroxyl group hydrogen-bonded to the polar head groups of the phospholipids and the hydrophobic skeleton is interacting with the nonpolar acyl chains of the lipids [4i]. Studying the process of vitamin D thermal formation from previtamin D in liposomes consisting of phosphatidylcholines of different carbon chain lengths, they came to the above hypothesis about stabilization of cZc-conformer [4i].
Provitamin D photoisomerization have been studied in water-ethanol mixtures in which steroids may form self-aggregates [4d,e]. The marked inhibition of tachysterol formation was observed at high water content whereas the increase of alcohol percentage turned the reaction in it's usual way. The effect has been explained by formation of dense packed steroid aggregates (B; Figure 3) in water-rich mixtures with hydrophobic cavity where the available conformational space is strongly restricted. In case of ethanol solutions with low water content aggregate A has been proposed, which allows internal rotation.
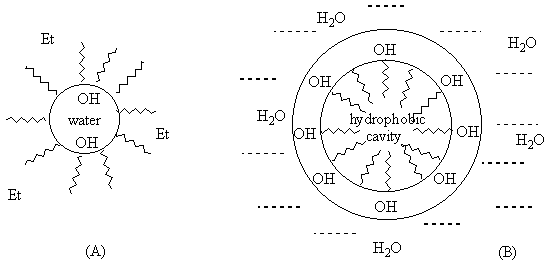
Figure 3. Representation of possible molecular aggregates of provitamin D in water-ethanol mixtures: (A) - ethanol is major component; (B) - water-rich systems
Cyclodextrines, cyclic carbohydrates of different oligomeric forms [11], have been numerously used as enzyme mimetics [12], drug delivery systems [13] and have served for molecular recognition studies[14]. Cyclodextrins have a truncated cone like shape with a hydrophobic cavity. These compounds have the ability to form inclusion complexes with organic molecules and modify the energetics and chemical reactivity of their guest molecules [15]. Naturally, these so popular and readily available systems have been employed as microenvironment for studying the photochemistry of provitamin D, vitamin D and its isomers. In particular, the vitamin D/▀-cyclodextrin inclusion complex have been studied in details [16,17]. Irradiation of provitamin D/▀-cyclodextrin complex [17] results in a significant inhibition of tachysterol formation, which is discussed in view of the conformational shift in favour of cZc conformation of previtamin D.
Photoisomerization of provitamin D have been also studied in thin film formed by evaporation of a chloroform solution on a glass slide [4a,b], on gel-bond (GB, an agarose gel support), polymer films ( poly-methylmethacrylate, PMMA; polyethylene, PE; polystyrene, PS) [4a,15] and CHOC liquid crystal film [18]. In the case of the provitamin D thin film on glass [4a] only previtamin D formation has been observed although at low conversion. In all the other cases, the reduction of tachysterol formation occurs depending on the polymer used. In [18], it is concluded that the decrease of free volume in this films is not large enough for selective synthesis of previtamin D, and that the effects of specific orientation or local interaction with the molecular assembly in films are of determining role. Interestingly to note that PMMA film, which is transparent in the UVA and UVB region, slightly reduces the efficiency of cis-trans isomerization in comparison with hexane solution (1. 47 times) in spite of the significant change in free volume. But in CHOC liquid crystal film the isomerization to tachysterol is favoured. Its accumulation at photostationary state is 1.17 times more than in hexane solution [18]. Unfortunately, in that study the different transparency of the systems have not been taken into account, which may significantly affect the active irradiation spectrum. This complicates a comparison. The strong inhibition of tachysterol formation found in polystyrene films which is similar like in toluene solutions may be attributed to a wavelength effect, by filtering out the short wavelengths around 254 nm so that the long wavelength irradiation around 313 nm becomes dominant.
In [4a], photolysis of provitamin D have been studied on silica gel (SG) TLC plates. Similar to the situation in liposomes, the photolysis on SG produces an increased steady-state product ratio of previtamin D to tachysterol. Silica gel adsorption stimulates irreversible degradation of the reaction products, which could not be prevented by standard experimental procedures. The effect of the kinetics of provitamin D photoisomerization by adsorption on silica gel surface have been studied in silica gel/solvent matrices [4c]. It has been suggested that adsorption of provitamin D and its photoisomers on the surface occurs via hydrogen bonding between the C3-hydroxyl-group of the steroid and surface active absorption centers ( surface water or Si-OH groups). Hydrophobic and electrostatic interactions restrict intramolecular rotation around previtamin D┤s single bonds and provide an orientation resulting in minimal contact of the steroids hydrophobic area with the water-saturated surface of the solid support. As a consequence rotation barriers are increased and previtamin D shifts its conformational equilibrium in favour of its cZc conformer (Figure 4).
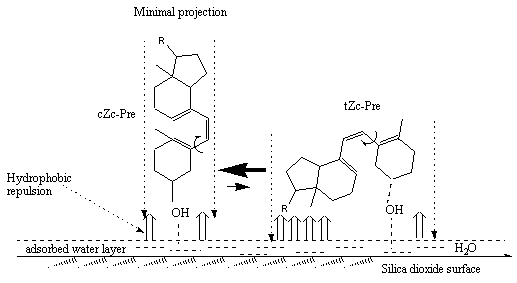
Figure 4. Pictorial representation of conformational shift caused by SiO
2 surface via hydrophobic repulsion between hydrophylic tail (R) and skeleton of previtamin D and water-saturated silica surface. Adsorbates of minimal imaginary projection on the surface should be mostly populated due to minimization of hydrophobic interactions. OH group of the steroid molecule is H-bonded to the active surface sites (coordinated water, surface hydroxyls [19])The effect by complexing previtamin D with silanol derivatives, formed via hydrogen bonds, on their conformational equilibrium has been calculated by use of MMX force-field (PCMODEL) in [20]. An increase in the population of cZc conformers with increasing the size of the silanol molecules (as a model for SiO2 surface) was observed. These calculations support the experimentally suggested conformational shift in previtamin D caused by the silica dioxide surface [4b-e].
III. Effects of UV-irradiation
Provitamin D and its main photoisomers absorb in the same UV-region (Figure 5) and being excited they all interconvert by photoisomerizations (Figure1). In addition previtamin D and tachysterol undergo irreversible conversions into toxisterols with different efficiencies. This results in complex reaction mixtures. The composition strongly depends on the wavelength of irradiation applied [21-23 and references therein].
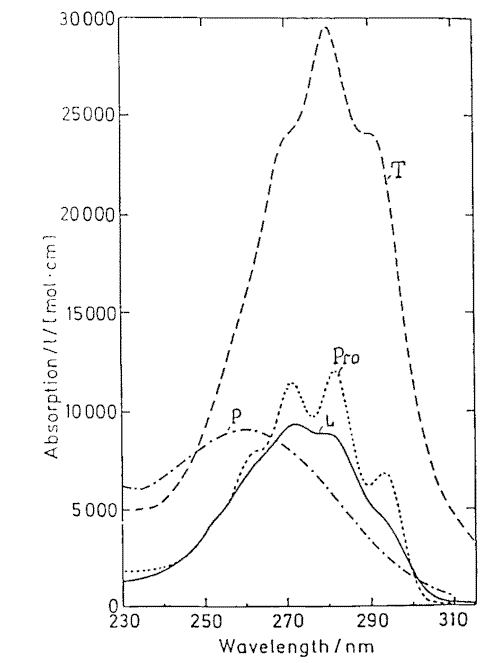
Figure 5. Absorption spectra of previtamin D photoisomers
Mainly, the wavelength dependence in previtamin D photosynthesis is caused by different absorbance of the photoisomers involved into reaction network and can be simulated by solving a system of kinetic equations [21-22]. It has been calculated and proofed experimentally that the optimum wavelength for previtamin D photosynthesis in solution is 296 nm [22a]. The experimentally derived action spectrum of previtamin D formation in human epidermis has the same maximum [2b]. This is of direct consequence for the optimization of the photochemical step in the industrial vitamin D synthesis [24-26] as well as for other applications like in UV-B-biodosimetry [27]).
There is another, more complicated wavelength effect in the previtamin D photochemistry. It arises from distinct changes observed in previtamin D photoreactions: a sudden increase in the efficiency of ring-closure reactions and the decrease of Z/E isomerization efficiency between 302 and 305 nm [22c-e] (see Table1).
Table 1. Quantum yields of previtamin D photoconversions
______________________________________________________
nm Pre-->Lumisterol Pre-->Pro Pre-->T ref.
______________________________________________________
253.7 0.04 0.014 0.41 [26]
302.5 0.09 0.02 0.29 [26]
0.09 0.04 0.27 [21d]
305.0 0.18 0.08 0.29 [21d]
______________________________________________________
Several models have been proposed to explain this dramatic changes in quantum yields :
1) ground state conformational control (selective excitation of different conformers possessing different absorption spectra and photoreactivity) [28]: Due to the high flexibility of the previtamin D┤s chromophore [8, 30] and its non-planar geometry [20, 29] the direct measurement of individual absorption spectra of conformers is unachievable and some experimental strategies like low temperature matrix techniques and computational separation of the individual spectra of the averaged solution spectra have been pursued [22c]. No marked difference between spectra of cZc conformer (the primary one formed after ring-opening from provitamin D) and the extended tZc conformers has been demonstrated experimentally [31]. Recent gas-phase semi-empirical calculations [29a,b] predict the difference in 0-0 transitions of cZc and tZc conformers, thereby strongly supporting the idea of the ground state conformational control as possible origin of the wavelength effect. cZc conformers can be selectively excited at the very red edge of previtamin D absorption band (which is actually a sum of individual conformer contributions), whereas tZc ones remain unactive.
2) involvement of different electronic states: This explanation involves the crossing of the
1B2 and 2A1 excited states [32]. It has been postulated that after being excited to the strongly allowed 1B2 state previtamin D may follow two routes of relaxation on its excited state potential energy surface - one is the direct path to the ground state surface of the E-isomer, the other is the decaying to the 2A1 state, which leads to ring closure. With wavelengths longer than 305 nm, the 2A state is directly excited and the channel to the E-isomer is no longer available. This postulate has been supported experimentally by fluorescence studies that involved numerous assumptions and mathematical treatments [32].3) hot reactions in the excited state: One of two competing isomerizations occur over a barrier in the exited state., The larger the energy of the excited molecule the more probable it will traverse this barrier making this pathway more efficient. This mechanism has been proposed to explain not only the sudden change of quantum yields (see above) but also their variation in the long-wavelength region [22c]. Nevertheless, the role of conformational control is not denied by authors [22c]. This model violates the NEER principle which does not seem unlikely in view of the recent postulate of a hula-twist isomerization mechanism (see below) [37].
IV. Theoretical models and molecular modeling
In order to explain experimental findings and to get a clear mechanistic picture on the previtamin D photoisomerization a number of different theoretical models and approaches have been studied. We will discuss them in accordance with the main focus of the model (mechanism, reaction kinetics, molecular properties):
1. Mechanistic representations
Conformational control or the NEER principle has served experimental chemists for many years as a more or less easy to follow practical guideline. This first and most simple concept successfully used in many experimental studies on conjugated trienes explains and predicts the outcome of such photoreactions [33]. Based on the non-equlibration of excited rotamers (NEER) [5a], and coupled with very short lifetime of the first excited state, one may expect the ratio of primary photoproducts to reflect the initial equilibrium composition of the ground state conformations. In this view (as we have already mentioned when describing studies on media-induced effects), the cZc conformation of previtamin D with its folded geometry would be a precursor of the ring closed products, while the tZc conformation leads predominantly to the trans-isomer tachysterol [7].
This mechanistic picture does not provide a view on the pathways of the molecules along the excited state surface up to the ground state, which started to be of increasing interest due to the development of ultrafast spectroscopy [34].
Work by Bernardi and coworkers [35] has suggested a new scenario that complements and unifies a large body of the experimental data. In contrast to the traditionally accepted model of avoided crossings between electronic states [36], there exist three crossings of the ground and excited states, termed conical intersections, through which the radiationless transition to the ground state surface may occur. These crossings take place at structures described as tetraradicaloid with three localized unpaired electrons along with a fourth unpaired electron that is delocalized in a quasi-planar allyl-like fragment. The broad variety of photorearrangement products in the previtamin D reaction network can be rationalized by the many possible spin couplings that can occur in the tetraradicaloid as it emerges toward the ground state surface at the conical intersection. The importance of conical intersections has been experimentally supported for other compounds [34 and references therein], and it is now a well established model among photochemists.
Recent low-temperature studies on previtamin D photo-chemistry undertaken by Fuss [10b] lead to new concept of the previtamin D Z/E isomerization: a "hula-twist" isomerization model. A decade ago, it has been proposed by Liu for the isomerization of polyenes, in particular for the retinal schiff base in rhodopsin [37]. This model of simultaneous twisting of a pair of adjacent double and single bonds strongly violates the NEER principle, as it was already mentioned before. Now new experimental evidences supporting this idea have appeared and recent work by Fuss [10b] is one example. In contrast to the earlier studies at low temperature [10a], the authors came to the conclusion that initially formed cZc-previtamin D undergoes E/Z isomerization into tEc-tachysterol through out-of-plane twisting motions of the C6-H and C7-H groups which requires significantly less volume than the complete rotation around the C6-C7 single bond. They claim that this process has no appreciable activation energy. Thus, these studies indicate also the fact that the cZc conformation can be a precursor of tachysterol too.
2. Modeling of the reaction kinetics
The widely used approach to the reaction kinetics modeling is based on the reaction model of provitamin D photoisomerization containing previtamin D as an intermediate isomer which can be reversibly photoconverted into provitamin D, lumisterol and tachysterol and irreversibly into toxisterols [20a-22]. The model is described by a system of rate equations (1a-e) and can be solved using standard mathematical methods.
dC
1/dt=A(f12e2C2 - f21e1C1) (1a)dC
2/dt=A[f21e1C1 + f23e3C3 + f24e4C4 - (f12 + f32 + f42 + f52)e2C2] (1b) dC3/dt=A(f32e2C2 - f23e3C3) (1c)dC
4/dt=A(f42e2C2 - f24e4C4) (1d)dC
5/dt=Af52e2C2 (1e)where A=J*l/(1-10
-D)/V*D, J is the incident radiation, D=l*S eiCi - optical density of the sample at the irradiation wavelength, ei and Ci are the extinction coefficient and concentration of ith-component, fij is the quantum yield for j--->i photoreaction (1 -provitamin D, 2 - previtamin D, 3 - tachysterol, 4 - lumisterol, 5 - toxisterols). V and l are the volume and path length of the cell.This approach has been successfully used for the determination of quantum yields [21], the predictions of their trends [4d-f], for the analysis of provitamin D photoreaction mixtures and for other applications [22-27].
Another modeling is based on scheme that includes also photoisomers in their singlet excited state and follows the NEER principle. It has been proposed by Fuss and coworkers [22b] in their nano- and picosecond laser studies, and was further generalized by Terenetskaya [38a] for the case of branched photoreactions with conformationally flexible intermediate [38b,c]. This modeling involves the analytical solving of the rate equations system, but since the rate constants of excited states population/depopulation are unknown (can be just roughly estimated), a number of assumptions and simplifications are used. Nevertheless, this approach provides an important basic information for the better understanding and/or predicting the certain external effects like intensity irradiation and ground-state conformational equilibrium shifts between two conformers considered [38]. For instance, it has been demonstrated that high intensity irradiation may result in the decrease of tZc conformer formation and, thereby, lead to the inhibition of tachysterol accumulation (which was found experimentally in [38d]).
3. Modeling of ground and excited states of previtamin D conformers
Knowledge about the properties of reactant ground and excited states is a necessary prerequisite for understanding the photoreaction mechanism. In view of the complex structure, flexibility and instability, the experimental information about previtamin D is often indirect or at all not available. Thus, the molecular modeling is of significant importance in the understanding and predictions of previtamin D photobehaviour. With development of computers and software, the computational studies on such large systems became now feasible even at high ab initio levels.
Previtamin D and its analogs have been studied by a variety of computational chemistry methods: molecular mechanics- [7,9,20,30,39,40], semi-empirical AM1- [30] and QCFF- [29], and ab initio calculations [29b,41]. All different theoretical approaches result in general in similar ground-state conformations with comparable geometries. In contrast to vitamin D and its isomers the ground state conformational preferences of previtamin D are highly sensitive to the theory applied in the computational studies. Nevertheless, the use of series of calculations within a certain approach may produce quite reliable relative tendencies and good agreement with the experiments [9,29,40]. In [20], for example, the results of MMX force-field calculations (PCMODEL[42]) on 10-desmethyl previtamin D analog show that absence of 10-methyl group reduce the preference for the cZc conformations in favour of tZc ones. This agrees well with the experimental observation of enhanced cis-trans isomerization of the 10-desmethyl analog [43] according to the NEER principle. In general, it should be noted, MMX force field optimizes very good geometries of the ring-closed provitamin D analogs (in contrast to the semi-empirical AM1 method) in comparison with X-ray structural data available [30].
The comparison of experimental results on provitamin D photoisomerization at 254 nm (when cis-trans isomerization is the prevalent photoprocess) in solvents of different polarity in conjunction with NMR data [7 and references therein] leads to the idea about the presence of larger population of cZc conformers with the C-3 hydroxyl group in equatorial orientation [9]. Concluding that polar solvent shifts axial-equatorial equilibrium in previtamin D thereby affecting cZc-tZc equilibrium in favour of the cZc conformation and therefore the efficiency of previtamin D cis-trans isomerization. MMX calculations show that cZc conformers are the most populated among the 3-OH equatorial series whereas tZc ones are prevalent when the 3-OH is axial oriented [9,20]. Recent ab initio calculations [29b] have established the same tendency: cZc conformers are more stable within the equatorial series then within the axial ones.
The change in treatment of intramolecular electrostatic interactions in MMX force-field calculations demonstrate an unique sensitivity of previtamin D conformational equilibrium in comparison with its other flexible isomers vitamin D and tachysterol. The MMX calculations with charge-charge and dipole-dipole approximations of the electrostatic interaction term lead to the inference of a very fine balance between steric and electrostatic interactions in previtamin D [30]. From these studies could be predicted that polar media (in which weaker electrostatic interactions develop) should depopulate tZc conformations. Again this agrees well with earlier findings [9].
The use of the semi-empirical QCFF/pi and QCFF/sol programs based on formal
s-p separability allowed to explore on the first excited singlet states of previtamin D conformers and thereby on their UV spectra [29]. It has been calculated that cZc conformers posses origin (0-0) transitions at longer wavelengths region than tZc ones. This result explains partially the effect of sudden change in quantum yields of the photoreactions at the red edge of their absorption band. At wavelengths longer than the origin transitions of tZc conformers, only cZc ones may absorb and undergo the photoconversions with their characteristic quantum efficiencies.The approach has been applied to the other previtamin D isomers (vitamin D, trans-vitamin D, tachysterol) and resulted in quite good agreement with the experimental UV-absorption characteristics for these compounds (see Table 2) [29b]. The spectral estimations on previtamin D were the most problematic ones because of discrepancies in ground state conformational preferences calculated at lower theoretical levels. But using ab initio (HF/6-31G) conformational analysis in combination with QCFF vertical transition calculations lead now to the best agreement with the experimental data (Table 2).
Table 2. Comparison of experimental (ethanol) and computational (QCFF) absorption characteristics for vitamin D, previtamin D and their E-isomers [29b].
compound |
f estd.(a) |
Maximum of absorption, nm |
Franck-Condon transition, nm |
f calcd. |
vitamin D |
0.60 |
265 |
273 |
0.61 |
5E-vitamin D |
0.76 |
271 |
278 |
0.77 |
tachysterol |
0.91 |
280 |
280 |
0.91 |
previtamin D |
0.28 |
260 |
246 - 269 cZc 252 - 268 tZc 257.5 (b) |
0.30-0.35 0.38-0.59 0.30 (b) |
(a)
Experimental oscillator strengths (festd.) have been calculated using formula festd.=extinction coefficient/31500 [44].(b)
The estimation is based on combination of QCFF results and 3-desoxy-previtamin D analog conformational distribution from HF/6-31G calculations.The dependence of ground-state conformational equilibrium of previtamin D on the theory applied distinguishes this steroidal triene as an unique flexible system even among it’s other triene isomers. The origin of the different conformational population results was located within a network of OH/
p interactions which are present in previtamin D and influences significantly its relative conformer stabilities in gas phase calculations [29b]. Empirical- (MMX) and semi-empirical (QCFF) methods assuming s-p separability are good for rigid systems or molecules where these interactions compensate each other and/or are of non-determining effect (e. g. global minimum is deep enough). But in the case of previtamin D they failed.Ab initio calculations also uncovered an effect of OH/
p hydrogen bonding interactions in previtamin D conformers with the 3-OH axial orientation [41a]. Since this modeling is aimed at the understanding of conformational behavior in solvents or in vivo (where the hydroxyl group is solvated or intramolecular hydrogen bonded and does therefore not interact with its p-system), the model of 3-desoxy-previtamin D analog should produce more reliable and with the experiments comparable results. This has been accounted in [29b] when the calculated UV absorption characteristics of previtamin D are compared with the experimental ones (Table2). Our latest calculations using Density Functional Theory (B3LYP/6-31G(d)) on 3-desoxy-previtamin D conformational distribution [41b] in conjunction with the experimental 0.7 kcal/mol destabilization of axial 1,2-dimethylcycloxene (a A-ring analog) in non polar solvents [45] produce now the best calculated value for the UV spectrum of previtamin D (lmax = 259 nm and fosc.=0.29) in accordance with the experimental one.V. Summary
Photosynthesis of previtamin D in mammal skin is an unique UV-induced photoprocess and the prerequisite for the thermal formation of vitamin D leading to a variety of important biological responds [46]. In contrast to the photoreaction in solutions, previtamin D photosynthesis in vivo is highly selective and efficient, that is making its mechanistic understanding of great interest for industrial purposes. This paper reviews experimental and theoretical models of previtamin D photosynthesis which mimic the in vivo and in vitro situation. The NEER principle, "hula-twist" and conical intersections in conjunction with experiments and molecular modelings appears to be key starting points for a better understanding of this process.
REFERENCES
1. (a) Biological Effects of Light, M.F. Holick and A.M. Kligman (eds.), 1992, Walter de Gruyter, Berlin - New York (b) S. Beissert and R.D. Granstein, (1996) UV-Induced Cutaneous Photobiology, Crit. Rev. Biochem. Mol. Biol. 31, 381-404;
2. (a) M.F. Holick, Photobiology of Vitamin D, in: Vitamin D, D. Feldman, F.H. Glorieux, J.W. Pike (eds.), 1997, Academic, San Diego, California, 33-39; (b) J.A. MacLaughlin, R.R. Anderson, M.F.Holick, (1982) Spectral Character of Sunlight Modulates Photosynthesis of Previtamin D and its Photoisomers in Human Skin, Science 216, 1001-1003; (c) M.F. Holick, J.A. MacLaughlin and S.H. Doppelt, (1981) Regulation of Cutaneous Previtamin D
3 Photosynthesis in Man: Skin Pigment is not an Essential Regulator, Science 211, 590-593; (d) R.R. Anderson, Tissue Optics and Photoimmunology, in: Photoimmunology, J.A. Parrish, M.L. Kripke, W.L. Morrison (eds.), 1983, New York: Plenum Press, 61-76;3. E. Havinga, (1973) Vitamin D, Example and Challenge, Experientia 29, 1181-1316;
4. (a) J.K. Yamamoto and R.F. Borch, (1985) Photoconversion of 7-Dehydrocholesterol to Vitamin D
3 in Synthetic Phospholipid Bilayers, Biochemistry 24, 3338-3344; (b) R.M. Moriarty, R.N. Schwartz, C. Lee, J.V. Curtis,(1980) Formation of Vitamin D3 in Synthetic Lipid Multibilayers. A Model for Epidermal Photosynthesis, J. Am. Chem. Soc. 102, 257-459; (c) I.P.Terenetskaya, O.G.Perminova, A.M. Yeremenko, (1990) Photoisomerization of Provitamin D in Dispersive Systems, J. Mol. Structure 219, 359-364; (d) I.P. Terenetskaya, O.G. Perminova, A.M. Yeremenko, (1992)Effect of Environment on the Conformational Equilibrium and Photoconversions of Previtamin D, J. Mol. Structure (Theochem) 267, 93-98; (e) I.P. Terenetskaya, O.G. Dmitrenko and A.M. Eremenko, (1995) Conformational Control in Previtamin D Photosynthesis by Heterogeneous Media, Res. Chem. Intermed. 21, 653-664; (f) O.G. Perminova, Comparative Studies on Provitamin D Photoisomerization in Homogeneous and Heterogeneous Media (in Russian), Ph. D. Thesis, Kiev, 1990; (g) X.Q. Tian, T.C. Chen, L.Y. Matsuoka, J. Wortsman, M.F. Holick, (1993) Kinetic and Thermodynamic Studies of the Conversion of Previtamin D3 in Human Skin, J. Biol. Chem.268, 14888-14892; (h) X.Q. Tian, T.C. Chen, Z. Lu, Q.Shao, M.F.Holick, (1994) Characterization of the Translocation process of Vitamin D3 from the Skin into the Circulation, Endocrinology 135, 655-661; (i) X.Q. Tian, M.F.Holick, (1999) A Liposomal Model that Mimics the Cutaneous Production of Vitamin D3. Studies of the Mechanism of the Membrane-Enhanced Thermal Isomerization of Previtamin D3 to Vitamin D3, J.Biol.Chem. 274, 4174-4179;5. (a) H.J.C. Jacobs and E. Havinga, (1979) Photochemistry of Vitamin D and its Isomers and of Simple Trienes, Adv. Photochem.11, 305-373; (b) H.J.C. Jacobs, F. Boomsma, E. Havinga, A. Van der Gen, (1977) Studies on Vitamin D and Related Compounds. Part XXVII. The Photochemistry of Previtamin D and Tachysterol, Rec. Trav. Chim. Pays-Bas 96, 113-117; (c) F. Boomsma, H.J.C. Jacobs, E. Havinga, A. Van der Gen, (1977) The "Overradiation Products" of Previtamin D and Tachysterol: Toxisterols, Rec. Trav. Chim. Pays-Bas 96, 104-112; (d) J.W.J. Gielen, The Photochemistry of Vitamin D and Related Conjugated Trienes, Ph. D. Thesis, Leiden, 1981; (e) A.G.M. Barrett, D.H.R. Barton, (1977) Photochemical Transformations. Part 34. Structures of Toxisterols, J. Chem. Soc. Perkin I 631-642;
6. P.A. Maessen, The Formation of Toxisterols from Previtamin D. Mechanistic Studies, Ph. D. Thesis, Leiden, 1983;
7. W.G. Dauben and D.J.H. Funhoff, (1988) Theorertical Evaluation of the Conformations of Previtamin D
3, J. Org. Chem. 53, 5070-5075;8. O.G. Dmitrenko, Conformational Flexibility in Previtamin D Molecule: Heterogeneous Media-Induced Effects, in: Spectroscopy of Biological Molecules: Modern Trands; P. Carmona, R. Navarro A. Hernanz (eds.), Kluwer Acad. Publishers, Dordrecht, 1997, p. 617-618;
9. O.G. Dmitrenko, I.P. Terenetskaya, W. Reischl, (1997) Solvent Effect on Previtamin D Conformational Equilibrium and its Photoreactions, J. Photochem. Photobiol., A: Chem. 104, 113-117;
10. (a) P.A. Maessen, H.J.C. Jacobs, J. Cornelisse, E. Havinga, (1983) Studies on Vitamin D and Related Compounds. Part 30. Photochemistry of Previtamin D
3 at 92 K. Formation of Unstable Tachysterol Rotamers, Angew.Chem. 95, 752-753; (b) A.M. Muller, S. Lochbrunner, W.E. Schmid, W. Fuss, (1998) Low-Temperature Photochemistry of Previtamin D: A Hula-Twist Isomerization of a Triene, Angew. Chem. Int. Ed. 37, 505-507;11. M.L. Bender, M. Komiyama, Cyclodextrin Chemistry, Reactivity and Structure, Concepts in Organic Chemistry, 6; Springer-Verlag: New York,1978;
12. R. Breslow, (1995) Biomimetic Chemistry and Artificial enzymes, Acc. Chem. Res. 28, 146-153;
13. K. Uekama, M. Otagiri, (1991) CRC Crit. Rev. Ther. Drug Carrier Syst. 3, 1-40;
14. M. V. Rekharsky, R.N. Goldeberg, F.P. Schwarz, Y.B. Tewari, P.J. Ross, Y. Yamashoji, Y. Inoue, (1995) Thermodynamic and Nuclear Magnetic Study of the Interaction of
a- and ▀- Cyclodextrines with Model Substances, J. Am. Chem. Soc. 117, 8830;15. A. Ueno, T. Osa, In: Photochemistry in Organized and Constrained Media, Ramamurthy, V. (ed), VCH Publishers, Inc., New York (1991) 739-782;
16. (a) J. Szejtli, E. Bolla, P. Szabo and T. Ferenczy, (1980) Pharmazie 35, 779; (b) J. Szejtli, Inclusion Compounds, J.L. Atwood, J.E.P. Davies, D.D. MachNicol (eds), Academic Press, New York 3 (1984) 445-471; (c) N.A. Bogoslovsky, B.I. Kurganov, N.G. Samochvalova, T.A. Isaeva, N.P. Sugrobova, I.E. Valashek and G.I. Samochvalov, Synthesis of 24R,25-Dihydroxyvitamin D from Vitamin D and Study on Inclusion Complexes of Vitamin D with ▀-Cyclodextrin in: Vitamin D. Molecular, Cellular and Clinical Endocrinology, Walter de Gruyter & Co., Berlin (1988)1021-1023;
17. X.Q. Tian and M.F. Holick, (1995) Catalyzed Thermal Isomerization between Previtamin D
3 and Vitamin D3 via ▀-Cyclodextrin Complexation, J. Biol. Chem. 270, 8706-8711;18. T. Majima, Y. Nakamura , T. Sato, (1991) Photochemical synthesis of a vitamin D analog in high selectivity, JPN Laser Sci.Research 13, 30-33;
19. (a) V.F. Kiselev, O.V. Krilov, (1978) Adsorption Processes on Semiconductor and Insulator Surfaces (in Russian), M., Nauka 256; (b) E.F. Sheka, I. Natkaniec, V.D. Khavryutchenko, P.B. Nechitaylov, A. Y. Musychka, V.M. Ogenko, I.V. Markichev, J. Brankowski, J. Krawczyk, Vibrational Spectroscopy of Dispersed Silica - Inelastic Neutron-Scattering (1990) J. Electr. Spectr. Rel. Phen. 54/55, 855; (c) I. Natkaniec, J. Fricke, V. Khavryutchenko, I. Makichev, A. Muzychka, V. Ogenko, G. Reichenauer, E. Sheka (1992) Water on Amorphous Silica - INS Study, Physica B 180, 522-524; (d) O.A. Kazakova, V. M. Gun┤ko, E.F. Voronin, S.S Sil`chenko, A.A. Chuiko, (1998) Interaction of Proteins with the Surface of Dispersed Silica in Aqueous Suspensions, Colloid Journal 60, 563-567;
20. O. Dmitrenko, and W. Reischl, (1996) Molecular Mechanics-Based Conformational Analysis of Previtamin D and its A-Ring Analogues, Monatshefte f. Chem.127, 445-453;
21. (a) K. Pfoertner, J.P. Weber, (1972) Photochemistry in the vitamin D series. I. Kinetics and Quantum Yields of Ergosterol Irradiation at 253.7 nm, Helv. Chim. Acta 55, 921-937; (b) K. Pfoertner, (1972) Photochemistry in the vitamin D series. II. Influence of the wavelengths on the photoisomerization of precalciferols, Helv. Chim. Acta 55, 937-947; (c) R. Mermet-Bouvier, E. Abilon, (1973) Ergosterol Photoisomerization Reaction Scheme, J. Pharm. Sci. 62, 891-894;
22. (a) M. Braun, W. Fuss and K.L. Kompa, Improved Photosynthesis of Previtamin D by Wavelengths of 280-300 nm, (1991) J. Photochem. Photobiol. A: Chem. 61, 15-26; (b) N. Gottfried, W. Kaiser, M. Braun, W. Fuss, K.L. Kompa, (1984) Ultrafast Electrocyclic Ring Opening in Previtamin D Photochemistry, Chem. Phys. Lett. 110, 335-339; (c) W. Fuss, S. Lochbrunner, (1997) The Wavelength Dependence of the Photochemistry of Previtamin D, J. Photochem. Photobiol., A.: 105, 159-164; (d) W.G. Dauben, P.E. Share, R.R. Ollmann, (1988) Triene Photophysics and Photo-chemistry: Previtamin D
3, J. Am. Chem. Soc.110, 2548-2554; (e) P.E. Share, Polyene Photophysics and Photochemistry: Previtamin D3, Ph. D. Thesis, Berkeley, 1985;23. (a) I.P. Terenetskaya, S.I. Gundorov, V.I. Kravchenko, E.B. Berik, (1988) Nanosecond Laser Photolysis of Provitamin D, Kvantovaya Elektron. 18, 1323; (b) N.A. Bogoslovsky, E.B. Berik, S.I. Gundorov, I.P. Terenetskaya, (1989) Characteristics of Laser Photolysis of Provitamin D, High Energy Chem. 23, 218; c) I.P. Terenetskaya, S.I. Gundorov, E.B. Berik, (1991) Characteristics of Photolysis of Provitamin D by Long-Wavelength Radiation, Kvantovaya Elektron. 21, 472; (d) Y.A. Repeev, I.P. Terenetskaya, (1991) Laser Photosynthesis od Previtamin D: New Effects of High-Intensity Picosecond Irradiation, Kvantovaya Elektron .23: 765-768;
24. Photochemistry, K. Pfoertner, Ulmann's Enciclopedia of Industrial Chemistry, VCH Verlagsgesellschaft mbH, D-6940 Weinheim, Vol. A 19 (1991) 573-597;
25. (a) I.P.Terenetskaya, N.A. Bogoslovsky, L.N. Vysotsky, F.I. Luknitsky, (1994) Routes to Optimization of Previtamin D Photosynthesis using Irradiation by Sunlamp, Pharm. Chem. J. 28, 589; (b) I.P. Terenetskaya, Y.A. Samojlov, N.A. Bogoslovsky, L.N. Vysotsky, F.I. Luknitsky, (1993) Pharm. Chem. J. 27, 797;
26. (a) W.G. Dauben and R.B. Phillips, (1982) Wavelength Controlled Production of Previtamin D, J. Am. Chem. Soc.104, 355-356; (b) V. Malatesta, C. W. Willis and P.A. Hackett, (1981) Laser Photochemical Production of Vitamin D, J. Am. Chem. Soc. 103, 6781-6783;
27. I.P. Terenetskaya, (1994) Provitamin D Photoisomerization as Possible UV-B Monitor: Kinetic Study using Tunable Dye Laser, Proc. of the Int. Conf. "Biomedical Optics '94", Vol.2134B, p.135;
28. (a) H.J.C. Jacobs, J.W.H. Gielen, E. Havinga, (1981) Effects of Wavelength and Conformation on the Photochemistry of Vitamin D and Related Conjugated Trienes, Tetrahedron Lett. 22 , 4013-4016; (b) A.M. Brouwer, J. Cornelisse, H.J.C. Jacobs, (1987) Wavelength Effect on the Photochemical Reaction of (Z)-2,5-Dimethyl-1,3,5-hexatriene, Tetrahedron 43, 435-438;
29. (a) O. Dmitrenko, W. Reischl, J.T. Vivian, J.H. Frederick, Ground and Excited States Properties of Previtamin D Conformers, Refered publication #C5 at Internet site: www.photobiology.com/ v1/contrib.htm, or www.photobiology.com/v1/dmitrenko/ dmitrenko.htm; (b) O. Dmitrenko, W. Reischl, J.T. Vivian, J.H. Frederick, Theoretical Studies of the First Strongly Allowed Singlet States of 3-desoxy Analogs of Previtamin D, Vitamin D and their E-Isomers, J. Mol. Struc. (Theochem), in press; (c) M. Nishino, M. Hirota (1989) CH/
p Interactions: Implications in Organic Chemistry, Tetrahedron 45, 7201-7245;30. O. Dmitrenko, W. Reischl, (1998) Modeling of Previtamin D Conformational Equilibrium: Effect of Intramolecular Electrostatic Interactions, J. Mol Struc. (Theochem) 431, 229-236;
31. A. Muller, Tieftemperatur-Photochemie von 7-Dehydro-cholesterol und Previtamin D, Diplomarbeit, Technical University of Munich, 1997;
32. (a) W. G. Dauben, P. E. Share, R. R. Ollmann Jr, (1988) Triene Photophysics and Photochemistry: Previtamin D
3, J. Am. Chem. Soc. 110 , 2548-2554; (b) W.G. Dauben, B. Disanayaka, D.J.H. Funhoff, B.E. Kohler, D.E. Schilke, B. Zhou, (1991) Polyene 2Ag and 1Bu States and the Photochemistry of Previtamin D, J. Am. Chem. Soc. 113, 8367-8374;33. H.J.C. Jacobs, (1995) Photochemistry of Conjugated Trienes: Vitamin D Revisited, Pure & Appl. Chem. 67, 63-70;
34. W. Fuss, S. Lochbrunner, A.M. Muller, T. Schikarski, W.E. Schmid, S.A. Trushin, (1998) Pathway Approach to Ultrafast Photochemistry: Potential Surfaces, Conical Intersections and Isomerizations of Small Polyenes, Chem. Phys. 232, 161-174;
35. F. Bernardi, M. Olivucci, I.N. Ragazos, M.A. Robb, (1992) A New Mechanistic Szenario for the Photochemical Transformation of Ergosterol: An MC-SCF and MM-VB Study, J. Am. Chem. Soc. 114, 8211-8220;
36. W.T.A.M. van der Lugt, L.J.Oosterhoff, (1969) Symmetry Control and Photoinduced Reactions, J. Am. Chem. Soc. 91, 6042-6049;
37. (a) R.S.H. Liu, D.T. Browne, (1986) A Bioorganic View of the Chemistry of Vision: H.T.-n and B.P-n,m Mechanism for Reactions of Confined, Anchored Polyenes, Acc. Chem. Res.19 , 42-48; (b) R.S.H. Liu, A.E. Asato, (1985) Photochemistry of Polyenes 22. The Primary Process of Vision and the Structure of Bathorhodopsin - A Model Study, Proc. Natl. Acad. Sci. USA 82, 259-263; (c) K. Schulten, P. Tavan, (1978) A Mechanism for the Light-Driven Proton Pump of Halobacterium halobium, Nature 272, 85-86; (d) R.S.H. Liu, D. Mead, A.E. Asato, (1985) Application of the H.T.-n Mechanism of Photoismerization to the Photocycle of Bacteriorhodopsin: A Model Study, J. Am. Chem. Soc. 107, 6609-6614; (e) R.H.S. Liu (1998) Looking Back to My Research Effort (1962-1998) www.bgsu.edu/departments/photochem/; Spectrum 11(4), 7;
38. (a) A.A. Serikov, I.P. Terenetskaya, (1994) The Influence of Radiation Intensity on the Pathways of Phototransformations of Conformationally Mobile Molecules, High Energy Chem. (USSR) 28, 257; (b) I.P. Terenetskaya, O.G. Dmitrenko, (1993) Theoretical Basis for the Possibility of the Conformational Control of the Products of Provitamin D Photochemical Conversion, Teoret. i Exper. Khim. 29, 326-332; (c) O.G. Dmitrenko, A.A. Serikov, I.P. Terenetskaya, (1996) Model Analysis of Branching Photoreactions with Conformationally Flexible Intermediate, J. Photochem. Photobiol., A: Chem. 96, 7-12; (d) I.P. Terenetskaya, Yu.A.Repeyev, (1991) Proc. Soc. Photo-opt.Instrum.Eng. 1403, 500
39. W.H. Okamura, M.M. Midland, M.W. Hammond, N.A. Rahman, M.C. Dormanen, I. Nemere, A.W. Norman, Conformation and Related Toplogical Features of Vitamin D: Structure-Function Relationships, in: Vitamin D. A Pluripotent Steroid Hormone: Structural studies. Molecular Endocrinology and Clinical Applications, A.W. Norman, R. Bouillon, M. Thomasset (eds), Walter de Gruyter, Berlin-New York (1994), 12-20;
40. O. Dmitrenko, W. Reischl, (1997) Ground-State Confor-mational Equilibrium of Previtamin D and its E-Isomer: Effect on the Absorption Characteristics, Res. Chem. Intermed. 23, 691-702;
41. (a) O. Dmitrenko, J.H. Frederick, W. Reischl, Ab Initio Study of Conformational Stability in Previtamin D, Vitamin D, and Related Model Compounds, J. Mol Struc. (Theochem), submitted; (b) O. Dmitrenko, W. Reischl, b3lyp/6-31G(d) calculations - unpublished results;
42. (a) PCMODEL (Serena Software, Bloomington, Indiana); (b) N.L. Allinger, H.L. Flanagan, (1983) Isotope Effects in Molecular Mechanics (MM2) Calculations on Deuterium Compounds, J. Comp. Chem. 4, 399-403;
43. R.B. Koolstra, J. Cornelisse, H.J.C. Jacobs, (1987) Photo-chemistry of Estra-5,7-diene-2▀,17▀-diol-17-acetate and its 9,10-seco Isomer, 10-desmethyl Analogues of Pro- and Previtamin D. 1. Reversible Isomerization Reactions, Rec. Trav. Chim. Pays-Bas 106, 526-533;
44. N.L. Allinger, J.C. Tai, Conformational Analysis 126. (1977) The Conformation and Electronic Spectra of Small Nonplanar Polyenes, J. Am. Chem. Soc. 99, 4256-4259;
45 J.B. Lambert, D.E. Marko, (1985) Factors Influencing Conformational Preferences in Cyclohexenes, J. Am. Chem. Soc. 107, 7978-7982;
46. R. Bouillon, W.H. Okamura, A.W. Norman, (1995) Structure-Function Relationships in the Vitamin D Endocrine System, Endocrine Rev. 16, 200-257;