Pressure pulses in the illuminated sample arise from the volume changes produced by radiationless relaxation (DVth) and structural solute-solvent rearrangements (DVr). Relaxation originates either from nonradiative decay of excited states or heat release (enthalpy change) in photoinitiated reactions. The structural volume changes reflect movements of the photoexcited molecules and/or the surrounding solvent in response to such events as dipole moment change, charge transfer, and photoisomerization. The overall pressure change is given by:
where p is the pressure change, V0 is the volume of the illuminated sample, kT is the isothermal compressibility. The pressure change induces an electrical signal H by the transducer, proportional to the pressure change through an instrumental factor k:
The voltage is given by:
Since the signal is proportional to the moles of absorbed photons, n (eq 4)
where El is the energy of an Einstein of wavelength l, a is the fraction of absorbed energy El released as heat q, DVe is the structural volume change per absorbed Einstein, cp is the specific heat capacity, r is the density, and b is the cubic thermal expansion coefficient of the solution (for dilute solutions these properties are those of the solvent). In order to obtain a and DVR, the instrumental constant, k', must be determined. By comparing the signal of the sample with that of a calorimetric reference, i.e., a system which releases all of the absorbed energy as heat in a time shorter than the instrumental response. The calorimetric reference and the sample solutions should be measured under identical experimental conditions such as solvent, temperature, excitation wavelength, and geometry. Since the isothermal compressibility kT is related to the isobaric compressibility, kS by:
care should be taken to use the proper values
of kT
in eq 3, especially when determining the thermoelastic
parameters by using a calorimetric reference in a different solvent. In
neat water
kT
= kS
and the situation is simpler. The signal
for the calorimetric reference is given by eq 7 (a
= 1)
In case permanent products or transient species
living much longer (ca 5 times) than the pressure
integration time are the only products of the photoreaction, the
amplitudes of the PA signals serve to determine
the heat and structural volume changes by applying eq 8 (obtained
from the ratio of eqs 7 and 5) relating the fluence-normalized LIOAS signal
amplitudes for sample and reference solutions, HSn
and HRn , respectively.
Numerical reconvolution has been used to retrieve the kinetic, enthalpic, and volumetric parameters, although direct deconvolution methods have been developed as well. The reconvolution methods assume a sum of single-exponential decay functions for the time evolution of the pressure (eq 10)
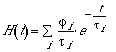 (10)
(10)where ti
is the lifetime of transient i and ji
is its fractional contribution to the measured
volume change. This assumption bears no implications
regarding the mechanism involved,
since several mechanisms lead to a pressure function
such as eq 10. A quadratic approximation
to the convolution integral has proven to be the most efficient
method to optimize the results carried out by means of a Levenberg-Marquardt
algorithm performing a least squares minimization.
The number of transients is successively increased
until no further significant (10-20%) improvement is observed in the value
of the root means square of the residuals and
in the residuals trend.
Up to three lifetimes have been resolved, provided
that they are separated by at least a
factor of 4. In the case of closer values,
a strong correlation between amplitudes and lifetimes
appears, preventing a reliable recovery of the
parameters. Lifetimes below a few nanoseconds
are integrated in the prompt response, for which
it is only possible to obtain the fractional
amplitude.
Lifetimes between a few nanoseconds and some
microseconds can be readily
deconvoluted. By applying simulations based on
a theoretical treatment of the signal,
restoration limits have been derived for amplitudes
and lifetimes in the case of a sum of two
single-exponential decays. The influence of variable
geometry, energy distribution, different
deconvolution methods, as well as that of the
signal-to-noise ratio on the restoration of the
amplitudes and lifetimes has been analyzed. Since
a general feature of the microsecond
transients is a strong correlation between their
lifetime and amplitude, their evaluation is more
critical. Simulations show that the front-face
geometry covers in principle a broader frequency
range than the perpendicular arrangement at the
same signal-to-noise level. However, the
polymeric detector used in this scheme has a
smaller sensitivity compared to the piezoceramic
detectors, which defeats the purpose of a higher
signal-to-noise ratio. The experimental
validation of these calculations has not been
performed as yet. The high absorbance needed
and other technical problems constitute a problem
with the front face geometry.
The fractional amplitudes ji in eq 10 contain the heat release qi and the structural volume change D VR,i of the i’th step. Both contributions can be considered additive provided that the time evolution of both is the same (eq 11). This assumption has been validated in several systems.
![]() (11)
(11)
where Fi
is the quantum yield of step i. qi
is related to the enthalpy of the species produced including the reorganization
energy of the medium and to the value of Fi.
D VR,i
represents the molar structural volume change of the i’th step, which includes
intrinsic changes in the chromophore as well as solute-solvent interactions.
Equation 8 is thus a particular case of eq 11 for i = 1.