| Papers and Posters | Site Home Page |
Following the Time Course Production of Dimethyl Telluride by Bacteria Using Fluorine-Induced Chemiluminescence
O. M. Akpolat and T.G. Chasteen
Abstract
The purpose of this study was to determine whether a facultative anaerobe, Pseudomonas fluorescensK27, would produce volatile organotellurium when anaerobic cultures amended with sodium tellurate or sodium tellurite. Batch bacterial bioreactor experiments were undertaken in order to observe the changes in the headspace of a growth media solution inoculated with Pseudomonas fluorescensand amended with tellurium salts. Gas samples were taken from the bioreactor every hour and were analyzed by capillary gas chromatography using fluorine-induced chemiluminescence detection to determine the composition of the headspace. Liquid samples were analyzed by a spectrometer to determine optical densities which were used as an indicator of cell growth. Different concentrations of tellurate and tellurite amendments were used. Verification of the identity of the dimethyl telluride produced in bacterial headspace above a tellurate-amended culture was achieved by comparison with the retention time of an authetic (CH3)2Te standard and by gas chromatography / mass spectrometry. The time course production of dimethyl telluride varied with Te amendment oxidation state and concentration. Higher Te concentrations caused slower bacterial growth but those cultures reached the stationary phase sooner than cultures amended with less Te. Mixed tellurite/tellurate amendment experiments exhibited a synergistic toxic effect and yielded less final biomass and very little DMTe production as compared to cultures amended with either tellurate or tellurite alone.
Toxic heavy metals are encountered in nature in many different forms. In air they are found as metal or oxide dust, in surface and ground water they are attached to humic substances and they are bound to soils and sediments as metal ions (Krishnamurthy, 1992). Some of these heavy metals, although toxic in significant amounts, are required in trace amounts by humans and animals. There is a small difference between the concentrations needed by the organisms for important catalytic processes and the concentrations where these metals become toxic. Biological methylation or biomethylation of these metals is an important mechanism in the transport of some of these metals in organisms.
Organometallic compounds with metal carbon bonds have quite
different properties than metal ions. The neutral organometallic compounds can usually
move across biological membranes as they tend to be lipid soluble (Hammond, 1980). They
remain intact through membranes which help them to be distributed in the system like any
lipid soluble compound. Methylation is the attachment of methyl groups to metals and
metalloids and is often responsible from the natural environmental mobility of these
elements (Thayer, 1989). Figure 1 is a schematic representation of the biomethylation
process. Here the ionic metal inorganic species are reduced and converted to
organometallic products by organisms.
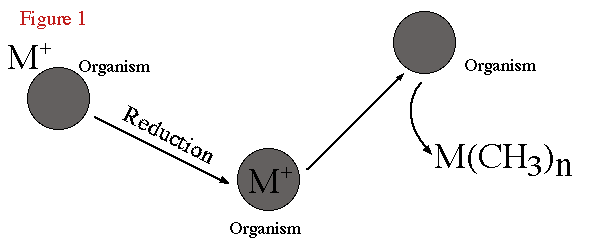 |
Figure 1. Schematic of biomethylation process. |
Methylation often leads to a change in the toxicity of the element. The conversion of selenate (Se+6) and selenite (Se+4) to volatile methylated species like dimethyl selenide and dimethyl diselenide by plants, microorganisms and mammals has been studied extensively (Challenger and North, 1934; Lewis et al., 1966; Chasteen, 1998). In the case of inorganic selenium, methylation by microorganisms can produce less toxic volatile organic compounds (McConnell and Portman, 1952; Sigel and Sigel, 1993). On the other hand methylated metals can be more toxic than their ionic forms from which they are derived. This comes from the higher solubility of these methylated species in lipid tissue thus having longer residence times in the organism. For example the half life of methylmercury in the human body is 70 days compared to 4-5 days for mercury(II) salts. Another way of measuring the lipophilicity of a compound is its distribution coefficient between water and 1-octanol (Wasik, 1978). The higher this value for a compound, the more lipophilic that material is thus the longer it will stay in the lipid tissue. These coefficients for HgCl2, CH3HgCl, (CH3)2Hg are 0.61, 2.54, and 191.3 respectively (Thayer, 1989).
The first notice of the methylation of inorganic compounds of tellurium occurred early last century. The odor exhaled by animals receiving inorganic derivatives of tellurium was first observed in 1824 (reviewed in Challenger, 1947). Later Hansen detected a garlic odor in the breath of dogs or men a few minutes after administration of potassium tellurite (Hensen, 1853). During his studies on the derivatives of tellurium at the University of Leeds, Challenger saw that this odor could easily be detected around those involved with the work; although they had never come into contact with organic compounds of tellurium. When Bird and Challenger analyzed the gasses evolved from test tube cultures of S. brevicaulis grown on bread containing potassium tellurite and they concluded that it was dimethyl telluride (Bird and Challenger, 1939). In the 1950s the horrible cases of "Minamata disease" (methylmercury poisoning) in Japan led into further investigations into this topic. Environmental applications utilizing alkylmetals have contributed to the development of this subject as well.
In 1945 Challenger suggested a biomethylation mechanism involving methionine. He showed that the biomethylation of arsenic involved some activated methionine intermediate which was later determined to be S-adenosylmethionine (Krishnamurthy, 1992; Chasteen, 1998). Challenger's proposed mechanism for selenium biomethylation involves four steps. In this mechanism, the selenium atom is methylated and reduced to form volatile dimethyl selenide (Challenger, 1945). The postulated intermediate selenium compounds were not detected in culture media, and it was concluded that the biological methylation process was confined to mould cells and did not take place in the medium. The mechanism is basically a transfer of a methyl group as a cation. One crucial compound missing in this proposed mechanism was dimethyl diselenide which has been observed by many workers in the recent years in the headspace above selenium resistant bacteria (Reamer and Zoller, 1980; Chasteen et al.,1990; Chasteen, 1993; McCarthy et al.,1993; Eriksen et al.,1999).
Reamer and Zoller (1980) broadened Challenger's proposed mechanism for the biomethylation of selenium based on the identification of dimethyl diselenide in purged sewage samples amended with selenium salts. This was a methyl carbonium pathway similar to that proposed by Challenger. They proposed a pathway for the intermediate CH3SeO2- to form dimethyl diselenide by reduction.
Later Doran (1982) suggested yet another mechanism for the same process. In his mechanism, selenite is first reduced to elemental Se and then further reduced to selenide which is methylated to dimethyl selenide. Evidence for this mechanism comes from the commonly observed elemental selenium in bacterial cultures doped with oxidized selenium salts (Francis et al., 1974; Doran and Alexander, 1977; Steinberg and Oremland, 1990; Eriksen, 1999).
Finally based on the observation that dimethyl selenenyl sulfide, CH3SeSCH3, was produced in some microbial cultures, Chasteen (1993) suggested that either chemical exchange or disproportionation could lead to the formation of CH3SeSCH3 when dimethyl diselenide, methanethiol and dimethyl disulfide are present in either cells or bacterial cultures. Since these organosulfur coumpounds are also biogenically produced in many microbial cultures, the entire process is inherently biological whether or not organoselenium is produced enzyamatically inside cells or by organosulfur exudates released into microbial culture.
It is thought that biological transformations for tellurium probably follow similar pathways as for selenium, which includes methylation and reduction by metalloid-resistant microbes. Fungi can also produce dimethyl telluride from tellurium salts as reported by Bird and Challenger (1939) and Chasteen et al., (1990). In monocultures of some photrophic bacteria amended with tellurate and most surprisingly elemental Te (powered metal), dimethyl telluride was detect in bacterial cultures after 7 days growing in the light (Van Fleet-Stalder and Chasteen, 1998). In other work, a strain of Penicilllium, which produced dimethyl selenide from inorganic selenium, was isolated from raw sewage. Dimethyl telluride was also produced by this organism from several tellurium salts. Although the proposed mechanism for methylating tellurium follows the same metabolic pathway as those for selenium and arsenic, dimethyl telluride was produced and detected only in the presence of selenium. The yield of dimethyl telluride was proportional to the input of inorganic selenium, so that no methylated tellurium compound was found until the input quotient of selenium to tellurium was about 10:1 (Jernelov and Martin, 1977). Thus the methylation of tellurium man not be a direct biological methylation, but rather a transmethylation from biologically formed methyl selenide compounds. Since the occurrence of tellurium in nature is rare, its environmental impact due to methylation is not expected to have significant effects. That said, recent expanded interest in the use of tellurium (Petragnani, 1994; Petragnani and Comasseto, 1991a;1991b) may increase its spread in the environment.
Very little is known on the biogeochemical cycling of tellurium. Alkylated forms of this element apparently form less readily under biogenic conditions than those of selenium. Although available data suggests that organotellurium compounds play a marginal role in the element's natural cycle, recently volatile compounds including dimethyl telluride, have been reported in gasses emitted by municipal waste deposits and landfill gas (Feldmann et al., 1994; Hirner, et al., 1994; Feldmann and Hirner, 1995), in geological settings (Hirner et al., 1998) and either volatile or particulate Te-containing species have been detected in atmospheric air though no speciation was done (Muangnoicharoen, 1989; 1990).
The biomethylation experiments reported here took place in a 3 liter
bioreactor using a facultative anaerobe grown in the presence of nitrate. Biomethylation
of tellurium salts by Pseudomonas fluorescens K27, a tellurium-resistant bacterial
strain which was isolated from Kesterson Reservoir in the San Joaquin valley of central
California (Burton et al.,1987) was observed and bacterial behavior monitored. Batch
cultures of K27 were amended with different concentrations of tellurium salts and were
grown in the bioreactor until they reached stationary phase. The growth of the bacteria
was monitored by optical density and the headspace was analyzed for reduced and methylated
tellurium compounds by using a fluorine-induced chemiluminescence detector after
separation by capillary gas chromatography. Calibration data for dimethyl telluride was
constructed from liquid, standard injections (acetonitrile solvent) and the concentration
of dimethyl telluride in the bioreactor's headspace gas was calculated knowing the
headsapce volume and using ideal gas assumptions.
Reagents
The reagents used throughout this research were analytical
grade chemicals and were used without further purification. Tryptic soy broth (TSB) was
obtained from DIFCO Laboratories (Detroit, MI USA). Sodium tellurate, Na2TeO4,
and sodium tellurite, Na2TeO3, were purchased from Aldrich Chemicals
(Milwaukee, WI USA). Potassium nitrate (certified A.C.S. grade) was ordered from
Fisher Scientific (Houston, TX, USA). Dimethyl telluride, CH3TeCH3
(DMTe), was procured from Organometallics, Inc., East Hampstead NH, USA and used as
received. Acetonitrile (Fisher Scientific, Houston, TX USA) was used to dilute DMTe for
the chromatographic calibration standards.
Growth Media
TSN3 medium (tryptic soy broth with 0.3 % nitrate) was
prepared by dissolving 10.0 g tryptic soy broth and 3.0 g potassium nitrate per 1.0 L of
deionized water. The freshly prepared growth media were sterilized by autoclave (15
min@121oC).
Standard Solutions
Tellurate Standard
A 20 mM stock solution was prepared by dissolving 1.904 g
sodium tellurate in 200 mL deionized water. This solution was filtered with a disposable
filter unit (Nalgene Company Rochester, NY USA) using a vacuum-pressure pump to sterilize
the standard.
Tellurite Standard
A 10 mM stock solution was prepared by dissolving 4.431 g
sodium tellurite in 200 mL deionized water. This solution was also filtered with a
disposable filter unit detailed above using a vacuum-pressure pump.
Bacterial Cultures
All the experiments were carried out using Pseudomonas
fluorescens K27. This is a metalloid-resistant bacterium harvested from Kesterson
Reservoir in the San Joaquin Valley of California, USA and isolated by Ray Fall at
University of Colorado, Boulder.
Instrumentation
The instrumentation used for the gas chromatographic
separation and chemiluminescence detection have been described elsewhere (Van Fleet-Stalder and
Chasteen, 1998). Briefly, a capillary gas chromatograph was interfaced to a gas phase
fluorine-induced chemiluminescence detector. A very thick film chromatographic column (30
m, 0.32 mm i.d. 5 µm 5% phenyl and 95% methyl polysiloxane film; J&W scientific,
Folsom, CA, USA) allowed us to chromatograph relatively low boiling point compounds (b.p.
>50oC) without having to use a cryogen and subambient oven temperature
programs. During some calibration experiments a split ratio of 1:50 was used to prevent
overloading the column or detector. The peak areas obtained from these injections were
therefore multiplied by 50 in order to normalize these peak areas to those of splitless
injections.
The following temperature programs were used to analyze samples and to check syringes. An 18 minute temperature program was used to analyze samples. The initial temperature was 30oC and was held for one minute, then a 20oC/min ramp was used up to 180oC; then a second ramp of 30oC/min was used up to the final temperature of 225oC, and the final temperature was held for 3 minutes. For syringe checks, a faster temperature program was used as there was no need to see good peak resolution (there shouldnít be any peaks in a clean syringe; any significant peak presence meant the syringe was not clean). In that program the initial temperature was 30oC, the ramp was 25oC/min up to 250oC and the final time was 2 minutes.
Bioreactor Experiments
The batch bacterial experiments were carried out in a New
Brunswick BioFlow III Batch / Continuous Fermentor (Edison, NJ USA). The fermentor
was disassembled, cleaned, reassembled, and filled with 2.5 L TNS3 media before every
experiment. It was then sterilized in a 716-liter autoclave (Wisconsin Aluminum Foundry
Co., Inc., Manitowoc, WI USA) for 15 min@121oC. The fermentor was connected to
a PC to record and/or control temperature, agitation, pH, and dissolved oxygen. The
fermentor had a condenser and a motor assembly connected to the top cover plate. It also
had a liquid and a gas sampling port. Gas samples were taken hourly using 1 mL gas-tight
syringes with push button valves, purchased from Alltech (Deerfield, IL USA) and were
analyzed using gas chromatography with fluorine-unduced chemiluminescence detection. Also,
liquid culture samples were taken hourly and optical density readings at 527 nm were
measured using a Spectronic 20D+ spectrometer.
Procedures
Experimental
Precultures of Pseudomonas fluorescens K27 were grown
aerobically at 30oC before the experiments. A few colonies of K27 were grown
aerobically in 50 mL TSN3 for at least a day then added to 200 mL more of TSN3. It was
then left to grow aerobically for another day. This was enough for the bacteria to reach
their stationary phase. This inoculation solution was then introduced into the fermentor
through one of its openings in the top plate (a 10% vol inoculum). After the fermentor was
purged with nitrogen to remove the dissolved oxygen in the system to make the bacteria
grow anaerobically, the batch was poisoned with the desired metalloid salts. The
fermentor, thermostatically maintained at 30oC, had metal fins rotating at 100
rpm that supplied good mixing. Liquid samples were taken from the batch every hour and
optical density and pH readings were recorded then.
Headspace Analysis
One mL gas samples were taken from the fermentor every hour.
The fermentor had a septum-lined gas sampling port where samples could be taken. After the
gas sample was pulled into the syringe, the push button valve was pushed to lock position
until the needle was inserted into the injection port of the GC. The valve was then opened
and the sample was injected rapidly. The valve was then pushed to lock once again and the
needle was pulled from the injector port. The GC head pressure gauge was checked after
every injection. A drop in the head pressure would mean a leak in the injectorís septum
and loss of sample during the injection.
After the injections were carried out, the syringes were taken to the cleaning device where they were left for an hour. It was sometimes necessary to rinse the syringes with acetonitrile before putting into the cleaning device to ensure perfect cleaning as determined by seeing only the baseline on the syringe check chromatograms. The cleaning device blew air into a closed flask which was heated on a hot plate. The approximate temperature of the hot plate surface was 75oC. The air then passed through the disassembled syringes which were also in the flask. After being cleaned in the cleaning device the syringes were checked by injecting 1 mL of lab air into the GC using the shorter syringe check temperature program. The cleaning process was repeated until no peaks were seen in the syringe check chromatograms.
Gas Chromatography/Mass Spectrometry
The headspace above a 0.1 mM tellurate amended culture of K27
was analyzed by gas chromatography/mass spectrometry, GC/MS (Saturn 3; Varian Associates)
to confirm the identity of the peak in our chromatograms that appear at approximately 7.3
minutes (see below). The column used in this analysis was a 30 m, 0.25 mm i.d., 0.25 mm
film, DB-5MS capillary column (J&W Scientific) with a carrier gas of UHP helium and a
head pressure of 12.5 psi used with splitless injection. The MS scan rate was 1
scan/second. Since no cryogen and subambient temperature program were available for this
instrument the separation of low boiling compounds was relatively poor compared to our
thick film capillary column used in the chemiluminescence method.
The unique photoelectrical and semiconducting properties of selenium have resulted in extensive use of this element in photocell devices and in xerography, solar batteries, specialty transformers and rectifiers. The principal commercial selenium compounds are mostly inorganic salts. Organoselenium compounds are mostly formed in living systems such as the protein derivatives, selenocystine and selenomethionine which have no significant commercial applications.Tellurium has similar applications in semiconductor industry and electronics, in the production of thermoelements, photoelements and other devices in automation equipment. The increasing demand for semiconductor use necessitates research work on the application of various tellurium compounds as semiconductors.
As with any chalcogen (S, Se, Te, Po), selenium and tellurium can be transformed from inorganic compounds to organometalloids through microbial activities (biomethylation of polonium has not be discovered yet). The resultant methylated compounds are more lipophilic and may be more volatile, thus changing their pattern of transport and possibly their toxicological behavior. Due to their limited usage, environmental contamination of tellurium compounds is not a serious problem. Environmental occurrence of organo-selenium and -tellurium compounds is still not well established due to the lack of suitable analytical methods, and their low concentrations in the environment. Although biomethylation of selenium has been investigated extensively, biomethylation of tellurium is not as well documented as the biomethylation of selenium. In fact very few data exist on the biomethylation of tellurium.
As with our previous work with this microbial strain, methylated
organo-sulfur compounds were routinely detected in the headspace of K27 growing on this
complex medium at 30oC. These compounds are biologically produced and released by this microbe into
headspace where gas concentrations vary depending on the growth phase (McCarty et al.,
1993). Figure 2 is an example of a tellurium amended culture early in log phase (2 hours
after inoculation and Te-amendment). Because of the kind of chromatographic column used in
this work, relatively low boiling compounds were not chromatographically resolved so only
dimethyl disulfide (DMDS; boiling point 109oC)
is identified in this figure (R.T. = 8.7 min).
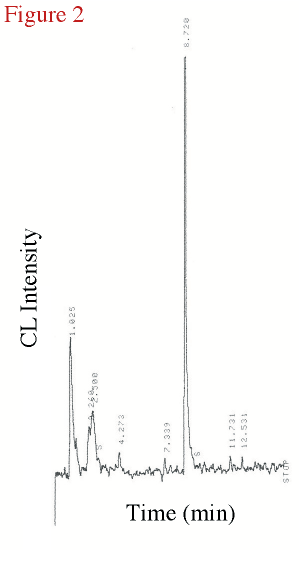 |
Figure 2. The chromatogram from a 0.01 mM tellurite amended bacterial experiment
taken 2 hours into the experiment. (Click on the image for a larger version.) |
Earlier headspace experiment with this same microbe more clearly detailed headspace production of methanethiol, dimethyl sulfide, and dimethyl disulfide (Chasteen et al., 1990; Zhang and Chasteen, 1994; McCarty et al., 1993). Also DMDS headsapce concentration was usually larger than dimethyl telluride by at least a (mole/mole) factor of 20 so at this point in the culture's time course (2 hours after inoculation, still in lag phase) only DMDS is present at any significant amounts (~200 ppbv). The earlier somewhat unresolved peaks are dimethyl sulfide and methanethiol based on correlations with our previous cryogenically trapped chromatography which better resolved earlier eluting peaks (Van Fleet-Stalder and Chasteen, 1998). Dimethyl telluride (b.p. 94oC) wasn't detected in large amounts until later in the log phase.
Figure 3 displays the chromatogram of the headspace from above a
0.01 mM tellurite-amended culture 25 hours after inoculation (in stationary phase). The
peak at approximately 7.35 min retention time in this Te-amended biological culture
mirrors the peak produced by a known standard of DMTe using this same temperature program.
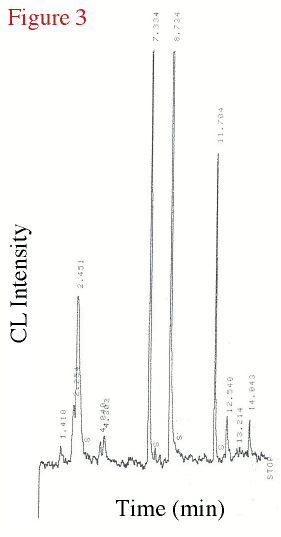 |
Figure 3. The chromatogram from a 0.01 mM tellurite amended bacterial experiment taken after 25 hours into the experiment, in stationary phase. |
In a further attempt to identify the compound eluting at 7.35 min in our chemiluminescence chromtography, GC/MS analyses using a different chromatographic column was carried out and the analyte boiling point region of the resulting total ion chromatogram produced an unresolved peak containing DMTe as determined by the mass spectrum. The difference mass spectrum (DMTe peak region minus nearby background) is shown in Figure 4 for the mass region from 120 to 170 daltons. The isotope pattern for tellurium can be seen in fragments for Te+, CH3Te+, and CH3TeCH3+. The three most abundant, stable, tellururium isotopes are 126Te (18.21%), 128Te (31.79%), and 130Te (34.48%).
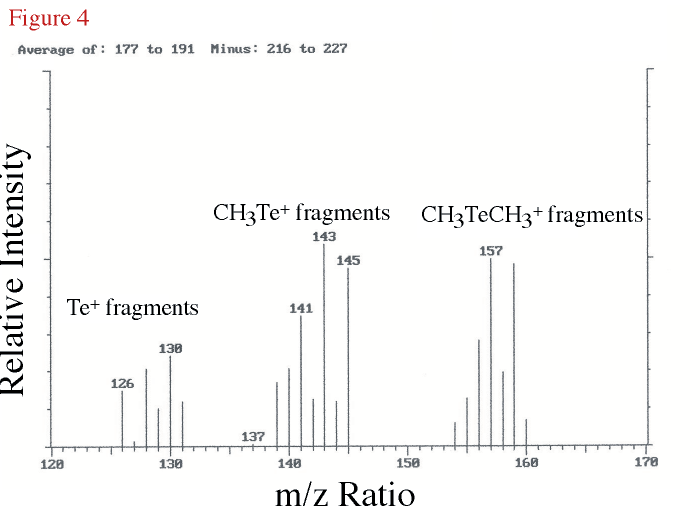 |
Figure 4. The difference mass spectrum for an unresolved DMTe peak showing only the
mass range from 120 to 170 daltons. The identity of the isotope grouping for DMTe
is indicated. |
Once we had established the biological production and identity of
DMTe and found that its concentration changed over time in the bioreactor headspace, time
course experiments involving different concentrations of tellurate and tellurite salts and
a mixture of both of these tellurium oxyanions were undertaken and the headspace sampled
through all three phases of growth. As the above chromatograms (Figure 2 and 3) show, DMTe
concentration increased over the time course of a batch cultures of this bacterium. Figure
5 shows the time course of DMTe, DMDS, and cell population of a K27 amended with sodium
tellurite.
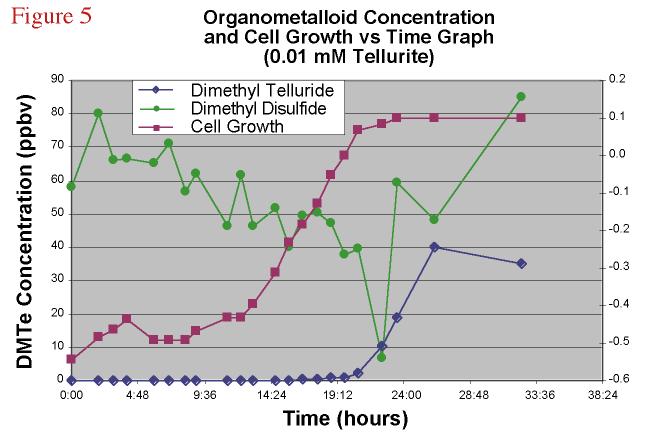 |
Figure 5. Dimethyl
telluride and dimethyl disulfide concentration and cell growth versus time graph for a
0.01 mM TeO3-2 amended bacterial experiment. |
Both of the compounds tracked in this figure show a change in concentration as the cell population increases with the DMDS decreasing as the culture reached log phase. DMTe conversely showed an increase only after log phase was reached and the headspace concentration leveled out as the culture reached stationary phase.
Tellurate-amended cultures (0.01 mM) showed roughly similar patterns
of growth (Figure 6) with the same overall final biomass as determined by optical density;
however, while this higher Te amendment concentration yielded about the same final
concentration of headspace DMTe, that DMTe production occured much later in the time
course, at the beginning of the stationary phase and the specific growth rate, as
estimated from the slope of the log phase, was less in the higher Te amendment culture.
This denotes the higher toxicity of a higher concentration of Te oxyanion.
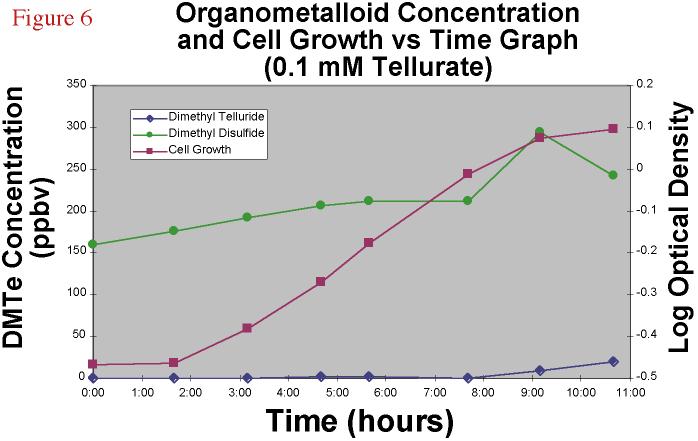 |
Figure 6.Dimethyl
telluride and dimethyl disulfide concentration and cell growth versus time graph for a
0.1 mM TeO4-2 amended bacterial experiment. |
Proceeding to even higher concentrations, 1.0 mM tellurate
amendments yielded even less DMDS and DMTe headspace, and the culture's log phase was less
distince and curved gently into stationary phase (Figure 7) after 11 or 12 hours.
Conversely, the 0.01 mM tellurite stationary phase was reached after 19 or 20 hours.
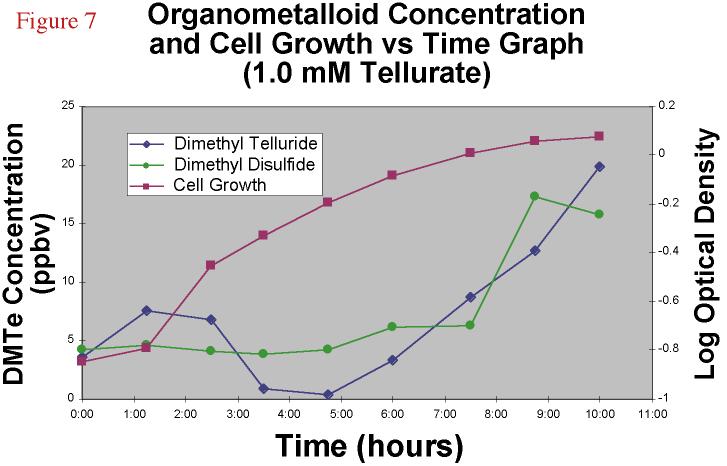 |
Figure 7. Dimethyl
telluride and dimethyl disulfide concentration and cell growth versus time graph for
a 1.0 mM TeO4-2 amended bacterial experiment. |
Noting that different concentrations and different oxidations state of tellurium oxyanions had substantially different effects on the growth and DMTe headspace production, we also carried out mixed amendment experiments: 0.01 mM TeO32- and 0.01 mM TeO42- added soon after inoculation, analogous to the previous experiment, yielded almost no DMTe production (< ~2 ppbv) and approximately half the final biomass of the individually amended experiments (either TeO32- or TeO42-). This highlights the synergistic toxic effects of tellurate and tellurite on this bacterium, a microbe that was isolated from a drainage system where both the oxyanions of this metalloid and many other metalloids and metals were present.
In conclusion, Pseudomonas fluorescens K27, when grown
anaerobicaly in 2.75 L volume batch cultures at 30oC and amended with either tellurite or tellurate, produced dimethyl telluride
which could be detected in the headspace. The time course production of DMTe varied with
Te amendment oxidation state and concentration. Higher Te concentrations caused slower
bacterial growth but those cultures reached the stationary phase sooner than cultures
amended with less Te. Mixed tellurite/tellurate amendment experiments exhibited a
synergistic toxic effect and yielded less final biomass and very little DMTe production as
compared to cultures amended with either tellurate or tellurite alone.
Bird, M.L.; Challenger, F.; J. Chem. Soc., 1939, 163-169.
Burton, G.A.; Giddings, T.H.; DeBrine, P.; Fall, R., Appl. Environ. Microb. , 1987, 185-188.
Challenger, F.; Chem. Rev., 1945, 36, 315.
Challenger, F.; Sci. Prog., 1947, 35, 396.
Challenger, F.; North, H.E., J. Chem. Soc., 1934, 68-71.
Chasteen, T.G., Appl. Organomet. Chem., 1993, 7, 335-342.
Chasteen, T.G.; In "Environmental Chemistry of Selenium"; Frankenberger, Jr. W.T. and Eugberg, R.A. Eds. Marcel Dekker: New York; Chapter 29; 1998.
Chasteen, T.G.; Silver, G.; Birks, J.; Fall, R. Chromatgr., 1990, 30, 181.
Doran, J., Adv. Microbial Ecol., 1982, 6, 17.
Doran, J.; Alexander, M.; Soil Sci. Sec. Am. J., 1977, 41, 70-73.
Eriksen, L., M.S. Thesis, Sam Houston State University, Huntsville, TX, U.S.A., 1999.
Feldmann, J.; Grumping, R.; Hirner, A.V., Fres. J. Anal. Chem., 1994, 350, 228-234.
Feldmann, J.; Hirner, A.V., Intern. J. Environ. Anal. Chem, 1995, 60, 339-359.
Francis, A.; Duxbury, J.; Alexander, M., Appl. Microb., 1974, 28,248-250.
Hammond, P.B.; Beliles, R.P. In "Toxicology"; Casarett, L.J.;Doull, J.; Macmillan: New York,1980, pp 409-467.
Hensen, A., Annalen., 1853, 86, 213.
Hirner, A. V.; Feldmann, J.; Goguel, R.; Rapsomanikis, S.; Andreae, M.O., Appl. Organomet. Chem., 1994. 8, 65-69.
Hirner, A.V.; Krupp, E.; Schulz, F.; Koziol, M.; Hofmeister, W., J. Geochem. Exp., 1998, 64, 133-139.
Jernelov, A.; Martin, A-L.; Ann. Rev. Microbiol., 1977, 33, 31-37.
Krishnamurthy, S.; J. Chem. Ed.,1992, 69, 347.
Lewis, B.; Johnson, C.; Delwiche, C., J. Ag. & Food Chem., 1966, 14, 638-641.
McCarty, S.L.; Chasteen, T.G.; Marshall, M.; Fall, R.; Bachofen, R., FEMS Lett., 1993, 112, 93-98.
McConnel, K.P.; Portman, O.W., Proc. Soc. Exp. Biol. Med., 1952, 79, 230-231.
Muangnoicharoen, S., 1989, Ph.D Dissertation University of Mussouri, Rolla, USA. Intern. Diss Abst. Int.B, 1990, 51, 176-177.
Petragnani, N.; Tellurium in Organic Synthesis; Academic Press: New York, 1994.
Petragnani, N.; Comasseto, J.V., Synthesis, 1991a, 794-817.
Petragnani, N.; Comasseto, J.V., Synthesis, 1991b, 897-919.
Reamer, D.; Zoller, W., Science, 1980, 208, 500-502.
Sigel, H.; Sigel, A., Eds. Metal Ions in Biological Systems, Vol. 29: Biological Properties of Metal Alkyl Derivatives; Dekker: New York, 1993.
Steinberg, N.; Oremland, R., Appl. Environ. Microb., 1990, 56, 3550-3557.
Thayer, J.S.; Appl. Organomet. Chem., 1989, 3, 123-128.
Van Fleet-Stalder, V.; Chasteen, T.G., 1998, J. Photochem. Photobiol., 43, 193-203.
Wasik, S.P. In Organometals and Organometalloids: Occurrence and Fate in the Environment; Brinckman, F.E.; Bellama, J.M., Eds., American Chemical Society: Washington, D.C., 1978, 314-324.
Zhang, L.; Chasteen, T.G., Appl. Organomet. Chem., 1994, 8, 501-508.
Acknowledgements
This research was supported by a Cottrell College Science Award of Research Corporation, the Texas Regional Institute for Environmental Studies, Sam Houston State University Research Enhancement Funds, and the Robert A. Welch Foundation.