| Papers and Posters | Site Home Page |
Chemiluminescence in the course of 4,4’-diazidodiphenyl photooxidation reaction
Elena N. Makareyeva a, Elena L. Lozovskaya b, Igor I. Sapezhinskii b,
Sergey V. Zelentsov a
a Nizhnii Novgorod State University, Chemical Department, 23 Gagarin Ave., Nizhnii Novgorod, 603600, Russia. Fax: +7(8312)658592, E-mail:
zelen@ichem.unn.runnet.ruElena N. Makareyeva a, Elena L. Lozovskaya b, Igor I. Sapezhinskii b,
Sergey V. Zelentsov a
a Nizhnii Novgorod State University, Chemical Department, 23 Gagarin Ave., Nizhnii Novgorod, 603600, Russia. Fax: +7(8312)658592, E-mail: zelen@ichem.unn.runnet.ru
b
Institute of Biochemical Physics of the Russian Academy of Sciences, 4 Kosygin St., Moscow, 117977, RussiaAfterglowing was observed after irradiation of 4,4’-diazidodiphenyl solution in ethanol with ultraviolet light with wavelength longer than 280 nm. We proposed that this effect is chemiluminescence of aromatic azide photooxidation products.
METHODS AND MATERIALS
4,4’-diazidodiphenyl (DA) was synthesized according to the standard method [1] and thrice recrystallized from ethanol.
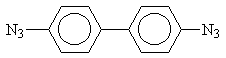
4,4’-diazidodiphenyl (DA)
The obtained DA has the following characteristics: Tm = 398 K, the ultraviolet spectrum has maximum at 293 nm (e = 26400 M-1cm-1), infrared spectrum has the picks at 2110, 2090, 1290 cm-1 that corresponds to literature data [2].
Chemiluminescence was observed with the apparatus shown in fig.1 that is similar to the one described in [3]. Solutions of DA in ethanol (volume 5 ml) were placed in to temperature-controlled cell (1) (T = 293 K). Irradiation was made with high-pressure mercury lamp DRK-120 (2) through the glass filter (3) that was transparent for irradiation with wavelength more than 280 nm. The reaction mixture was constantly stirred. The irradiated solution was pumped to the cuvette (5) of the photometric installation equipped with high sensitive photomultiplier (6) (FEU-36 spectral sensitivity region is 300-600 nm, minimal intensity of chemiluminescence that can be measured is about 3´ 102 photons´ ml-1´ s-1) to record the emission. Time of pumping was less than 1 s. The irradiation of the ethanol used as a solvent alone did not produce the emission.
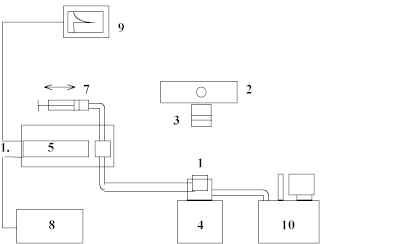
Figure 1. Photochemiluminescence measurement system: 1 – temperature-controlled cell, 2 - high-pressure mercury lamp DRK-120, 3 - glass filter, 4 - magnetic stirrer, 5 – cuvette of the photometric unit, 6 - high sensitive photomultiplier, 7 – pumping unit, 8 – high voltage power supply, 9 – recorder, 10 - thermostat.
Results
The curve of the afterglowing of the irradiated DA solution is shown in fig.2. Just after pumping of the irradiated DA solution into the cuvette for measurement it was registered afterglowing. The initial part of the decreasing curve can be described by exponential law with t e about 5 s. After approximately 20 s from the moment of pumping the chemiluminescence intensity began to rise, then passed through flat maximum at t = 30 – 60 s, and after that slow decreased again. It was revealed that an appearance of the second maximum on the afterglowing curve depended on irradiation time. Really we recorded the second maximum at tirrad = 1 min. When the irradiation time was either 30 s or 75 s and more the afterglowing curves had the ordinary shape and the second maximum was not observed.
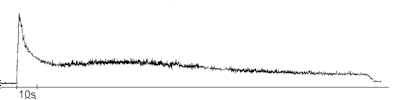
Figure 2. The afterglowing curve of the DA solution in ethanol (0,13 mM) irradiated with light longer than 280 nm, tirrad = 1 min.
Moreover, for longer periods of irradiation we found the abrupt increase in chemiluminescence intensity in the first maximum region (see Table).
Table. Effect of irradiation time on chemiluminescence intensity at photooxidation of 4,4’-diazidodiphenyl solution just after pumping into cuvette for measurement.
Time of irradiating, s |
Chemiluminescence intensity, relative units |
30 |
30 |
60 |
75 |
75 |
540 |
90 |
>2000 |
On the basis of the obtained results it is possible to make some preliminary conclusions. Some products of the DA photooxidation (at least two) may be formed in the excited states so they can emit chemiluminescence. Formation of both emitting intermediates is strongly depending on irradiation time. As the second pick was observed at the irradiation time about 1 min it was possible to propose that the second excited product was formed as a result of absorption followed by photochemical reaction of one of the intermediates of the DA photooxidation which life-time was in the interval 30 < t < 75 s.
DISCUSSION
The azide photooxidation reaction was the subject of some works [4-6]. The first step of the process is the azide group photolysis to produce nitrene and eliminate molecular nitrogen. The chemiluminescence observed may be emitted by the particles in the excited state. They can be formed as a result of (i) Triplet-triplet annihilation of the triplet nitrene and molecular oxygen [5];
(ii) Destruction of the tetraoxide [5,6]:
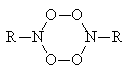
(iii) A reaction of the type
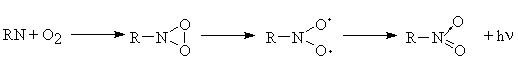
Thus at the present on the basis of the data obtained it is impossible to make definitive conclusion about concrete products that are responsible for appearance of the chemiluminescence emission. This problem needs to be studied in more detail. Owing to its high sensitivity, the chemiluminescence method is very suitable for studying primary photochemical reactions and the role of various active intermediates involved in the photooxidation reaction and taking part in chemiluminescence. Further studies of the chemiluminescence accompanying the DA photochemical reaction can be essential for understanding of the photooxidation mechanisms of aromatic azides.
References
Biffin, M.E.C.; Miller, J.; Paul, D.B. The chemistry of the azido group. Ed. By S. Patai. Intersci. Publ. London e.a. 1971, 57-190.
Yurst, J.E. The chemistry of the azido group. Ed. By S. Patai. Intersci. Publ. London e.a. 1971, 191-202.
Sapezhinskii I.I. Biopolymers: Kinetics of Radiation and Photochemical Conversions, Nauka: Moscow, 1988.
Abramovitch, R.A.; Challand, S.R. J. Chem. Soc., Chem. Comm. 1972, 16, 964-966.
Brinen, J.S.; Singh, B. J. Am. Chem. Soc. 1971, 93, 24, 6623-6629.
Pritchina, E.A.; Gritsan, N.P. J. Photochem. Photobiol. A.: Chemistry. 1988, 43, 165-182.