| Papers and Posters | Site Home Page |
UV AND VISIBLE LIGHT – INDUCED MUTATIONS IN ESCHERICHIA COLI
K. Sh. Voskanyan
Department of Radiobiology, Joint Institute for Nuclear Research, 141980 , Moscow Region, Dubna, Russia.
ABSTRACT The induction of Lac- mutations in Escherichia coli K-12 (Hfr) under 216, 270, 532 and 633 nm laser irradiation has been investigated. In all the cases, irradiation has lethal and mutagenic effects on bacteria, and all the mutagenic curves are of a similar form: in the region of small irradiation doses a peak of mutation frequency is observed, further the curve is reduced and again increases at greater irradiation doses. Taking into account that non-irradiated E.coli bacteria cells are capable of filament formation, we can suppose that the mutation frequency curve consists of two components: component of more sensitive filaments ( low dose region ) and component of normal cells ( high dose region).
Keywords: Bacteria, Lac- mutations, uv and visible light. |
- INTRODUCTION
- MATERIALS AND METHODS
- RESULTS AND DISCUSSION
UV light has been shown to be lethal and mutagenic in a variety of organisms, including bacteria [1, 2, 3, 4, 5].
It is generally considered that cyclobutane pyrimidine dimers and pyrimidine-pyramidone or [6-4] photoproducts are most important premutational DNA lesions induced by UV radiation [6, 7, 8, 9, 10, 11, 12]. Other lesions such as DNA strand breaks and thymine glycols are also induced by UV treatment [13, 14].
The correlation between the quantity of energy absorbed by DNA and the observed biological effect (survival, mutation frequency) is illustrated in the 254-320 nm wavelength region [15].
At wavelengths above 320 nm the radiation effect on DNA has a mediated, most probably, a photodynamical character.
The representations made in Witkin’s papers [16, 17, 18, 19], according to which mutations appear only due to the errors made in SOS repair system, are widely accepted.
We must also note that the literature data of mutation induction frequency dependence on irradiation dose are contradicting. On the basis of the results obtained by the majority of the authors of UV-induced mutagenesis, we may conclude that the frequency of UV induced mutagenesis depending on the dose is quadratic or near to it [20, 21, 22, 23, 24]. However, curves of the following types are also observed: growth-plateau (maximum)-slope [ 25]. All the existing data on induced UV-mutagenesis are obtained by using radiation with the wavelength l > 240 nm. Though the 240-185 nm range is of special interest as the absorption of protein peptide bonds, as well as the one of the DNA absorption peaks are located in this region. Nowadays, the fulfillment of similar experiments have become possible due to the development of lasers [26, 27].
Till recently, it was considered that the visible light has no mutagenic effect on cells. Nowadays the possibility of visible light induced mutations is shown in [28, 29].
Besides, on the basis of our previous findings on the lethal effects of 633 nm laser irradiation on E.coli K-12 [30, 31] it is expected that laser induced cell inactivation is due to the induction of DNA damages (similar to ionizing radiation). Since ionizing radiation gives rise to lethal effects accompanied by mutations due to induction of DNA damage, we also tested the induction of mutations by red light in order to show a possible induction of damage to DNA.
The 633 nm light is not absorbed by DNA, and the observed effects may be due to an indirect action. Cellular cytochromes belonging to the respiratory system and showing maximum absorption in this spectral range may constitute the primary photoreceptors. This possibility has been put forward in a recent review [32]. If so, similar photobiological effects should be observed by irradiating cells with green light, which is also absorbed by cytochromes [33].
The present investigations were carried out with the aim to find out the mutagenic action of the 216, 270, 633 and 532 nm laser radiation on bacterial cells. The results are of interest also because of the wide-spread use of lasers in medicine [34, 35].
Helium-Neon (He-Ne) laser (LG-75, Lvov, Ukraine) of continuous action (CW) with wavelength l = 633 nm, radiation power 10 mW and quasi CW laser (LTI – 720, Saratov, R & D Co. "Polyaron", Russia), l = 532 nm, pulse duration t = 175 ns, were applied for cell irradiation.
As a source of UV laser radiation we employed a picosecond Yttrium alluminate: Nd laser with two amplifiers and frequency conversion into the fourth (l = 270 nm) and fifth (l = 216 nm) harmonics. The UV radiation parameters were as follows: the energy of a single pulse
E = 0,1-1 mJ, pulse duration t = 15 ps, and repetition rate ¦ = 2 Hz.
Before irradiation, cells were grown on solid complete nutrient medium PPA (plain peptone agar from Gamaleya Institute of Microbiology, Moscow, Russia) during 24 hours at 37° C. Irradiation of cells was performed on solid agar (4% agar) without nutrients, in a monolayer at room temperature.
The experiments were done with strain Hfr of Escherichia coli K-12 .
Cell survival was defined by the macrocolony method [36]. To express the mutated cells, we applied the "sandwich seeded" method [37]: irradiated samples were washed from the pieces of solid agar with physiological solution, and were seeded in Petri dishes with 12 ml PPA (in which we added 50 ml threpheniltetrazoliumchloride before autoclaving, and 50 ml of 20% lactose, one of selective medium after it) and then we also filled in 12 ml of the same medium. Mutated colonies were red. The counting of the mutant colonies was carried out with the help of a MBC-9 microscope (" LOMO" Co., St. Petersburg, Russia).
The mutation frequency was measured as a ratio of a mutant clone (Nm) to the number of surviving cells (N).
The number of spontaneous mutations determined by this technique in the control (non-irradiated) culture was equal to 10-7 cells on average.
5-7 independent experiments were performed, and the standard errors for each experimental point were lower than 5%.
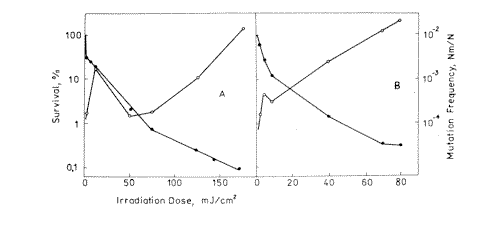
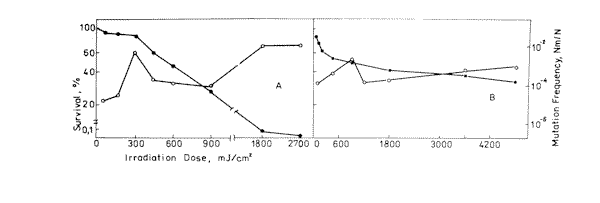
Experimental values of mutagenic and lethal effectiveness of 216, 270, 532 and 633 nm laser radiation are shown in Figures 1 and 2. It is obvious from the figures that in all the cases irradiation had lethal and mutagenic effects on bacteria, and that all mutagenic curves are of similar form: in the small irradiation region the peak of mutation frequency is observed, further the curve is reduced, and at a greater irradiation dose it increases again. Probably, such a similarity is an evidence of the fact that the character of the induced mutagenesis curve is defined neither by primary photoreceptors, nor by certain photodecompositions that cause mutations, but by phenomena that are common for all irradiation cases. Curves with maximum mutation frequencies are well known in the case of cell irradiation with UV or ionizing radiation [38, 39]. It is assumed to be connected with the great probability of mutated cell destruction. In our case, effect of such kind is observed in the range of low doses. It is well known, that non-irradiated E. coli bacteria cells are capable of filament formation. Its frequency depends on cell cultivating condition. In the case of our experiments (grown on solid complete medium at 37° C) the frequency of filament formation can arrive to 30% [40]. It was considered that fil+ gen in E.coli cells doesn’t affect DNA reparation processes, but increases its sensitivity to radiation [40,41]. Taking all this into account, we can suppose that the mutation frequency curve consists of two components: a component of more sensitive filaments (low dose region) and a component of normal cells (high irradiation dose). For the filaments, the mutation frequency curve has a shape with maximum, but for the normal cells we observe near to linear-quadratic dependence on dose exposure. The form of the cell surviving curves, which indicate heterogenity of the irradiated culture, also testifies in favour of such suppositions. It was shown on bacteria cells that the nature of mutation frequency curve depends a lot on expression specificities of SOS-cell system of inducible genes [42]. The same conclusion was obtained for mutagenic effect study at the UV radiation action on bacteria cells [17,18,19]. Consequently, the dependence of the bacterial cells mutation frequency on dose is much connected on the cells reparation system. Thus, it is possible that the linear-quadratic dependence of mutation frequency on dose refers to the error-prone component of reparation system. Numerical characteristics of these curves are given in Table 1. It is shown that the 270 nm radiation has the greatest lethal effect, and the 216 nm radiation has the most effective mutagenic effect. The first one is connected with the fact that the 270 nm light is mostly absorbed by the DNA molecules, and as it is accepted by photobiology, DNA damages are the basic cause of cell destruction. The 216 nm radiation was absorbed by DNA molecules as well as the proteins, and in this case the dimer output sharply decreases compared with irradiation at 270 nm [43]. If so, it is possible that not only dimer formation is the basic damage responsible for mutation development at 216 nm. The observed mutagenic effect at 216 nm can be mixed with nucleic and protein components. It is very difficult to explain the mechanism of mutation induction in the visible range, as the absorption mechanism itself and mastering of the incident light are unknown. The most probable candidates for the visible light absorption in E.coli are terminal oxidase cytochromes 0 and a [33]. Thus, the primary photoacceptors for UV light are nucleic acids and proteins, and visible light is absorbed by cytocromes. Therefore different primary photoacceptors for UV and visible light in E,coli cells are responsible for different lethal and mutagenic actions of these two spectral regions of light. The damage of nucleic acids influences the viability of a cell much more strongly than damage to respiratory chain. If we calculate the obtained dose value for the survival and mutation frequency per one quantum of incident radiation and insert the results in Figure 3, taken from paper [15], we can see that the comparative survival and mutation frequency at 270 nm wavelength in our case also correlates with the DNA absorption and thymin dimer output. However, at less than 254 nm and more than 320 nm this correlation disappears.
Figure 1. Survival ( l ) and mutation frequency ( m ) curves of bacteria cells at 270 nm ( A ) and 216 nm ( B ) light irradiation.
|
Figure 2. Survival ( l ) and mutation frequency ( m ) curves of bacteria cells at 633 nm ( A ) and 532 nm ( B ) light irradiation.
Table 1. Parameters of survival and mutation frequency curves. Dm- irradiation dos for 10-3 mutation induction. LD50 – irradiation dose corresponding to 50% survival. |
l nm |
LD50 mJ/cm2 |
Dm mJ/cm2 |
216 |
3 |
26,5 |
270 |
1 |
130 |
532 |
2× 102 |
32× 102 |
633 |
6,4× 102 |
16× 102 |
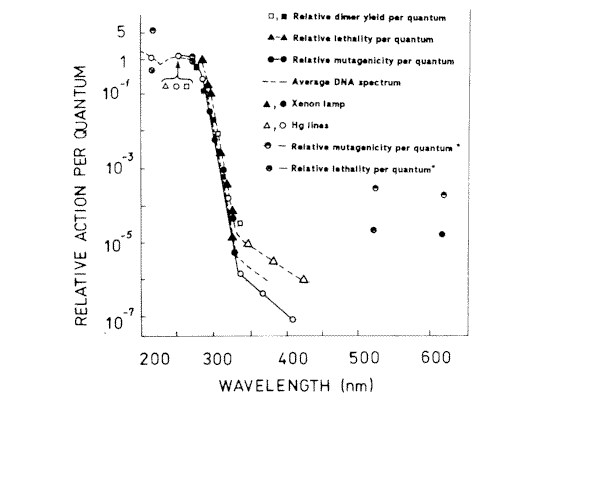
Figure 3. Action spectra for lethality, and mutagenesis in E.coli WP2s, and cyclobutyl pyrimidine dimer induction in E.coli RT4. The lethality data points were calculated from F 37 values though the spectrum, and the mutagenesis data points were calculated from the dose required to reduce 3x 10-6 revertants per survivor. (*) – our results
REFERENCES |
- Auerbach C. Mutation Research, Chapman & Hall, London, 1976.
- Witkin E.M. Ultraviolet mutagenesis and inducible DNA repair in Escherichia coli. Bacteriol. Rev. 40 (1976) 869-907.
- Bernstain C. Deoxyribonucleic acid repair in bacteriophage. Microbiol. Rev. 45 (1981) 72-98.
- Haynes R.H. and Kunz B.A. DNA repair and mutagenesis in yeast. In The Molecular Biology of Yeast Saccharomyces. Life Cycle and Inheritance, in Strathern J.N., Jones E.W. and Broach J.R. (Ed.) Gold Spring Harbor Laboratory Press, New York. 1981, pp. 317-414.
- Friedberg E.C. DNA Repair ,(Ed.) W.H.Freeman, New York 1985.
- Kunz B.A. and Glickman B.W. The role of pirimidine dymers as premutagenic lesions: a study of targeted vs untargeted mutagenesis in the lac1 gene of Escherichia coli. Genetics. 106 (1984) 347-364.
- Mitchel D.L., Haipek C.A. and Clarkson J.M. [6-4] photoproducts are removed from the DNA of UV-irradiated mammalian cells more efficiently than cyclobutane pyrimidine dymers. Mutat. Res. 143 (1984) 109-112.
- Wood R.D., Skopet T.R. and Hutchinson F. Changes in DNA base sequence induced by targeted mutagenesis of lambda phage DNA by ultraviolet light. J.Mol.Boil. 173 (1984) 273-291.
- Franklin W.A., Doetsch P.W. and Haseltine W.A. Structural determination of the ultraviolet light-induced thymine-cytosine pyrimidine-pyramidone [6-4] photo-product. Nucleic Acid Res. 13 (1985) 5317-5325.
- Glickman B.W., Schaaper R.M., Haseltine W.A., Dunn R.L. and Brash D.E. The C-C [6-4] UV photoproduct is mutagenic in Escherichia coli. Proc. Natl. Acad. Sci.USA. 83 (1986) 6945-6949.
- Protic-Sabejic M., Tuteja N., Munson P.J., Hauser J., Kramer K.H. and Dixon K. UV light-induced cyclobutane- pyrimidine dimers are mutagenic in mammalia cells. Mol. Cell. Biol. 6 (1986) 3349-3356.
- Brockrath R., Ruiz-Rubio M. And Bridges B.A. Specificity of mutation by UV light and delayed photoreversal in umuC-defective Escherichia coli. K-12, a targeting intermediate at pyrimidine dimers. J.Bacteriol. 169 (1987) 1410-1416.
- Hariharan P.V. & Gerutti P.A. (1977) Formation of products of the 5-6 dihydroxy dihydrotymine type by ultraviolet light in HeLa cells. Biochemistry 16 12 (1977) 2791-2795.
- Miguel A.G. and Tyrrell R.M. Induction of oxygen-dependent lethal damage by monochromatic UVB (313) radiation: strand breakage, repair and cell death. Carciogenesis, 4. (1983) 375-380.
- Peak M.J., Peak J.G., Moehring M.P. and Webb R.B. Ultraviolet action spectra for DNA dimer induction, lethality, and mutagenesis on the UVB region. Photochem. Photobiol. 40 (1984) 613-620.
- Witkin E.M. Radiation-induced mutations and their repair. Science, 152 (1966) 1345-1353.
- Witkin E.M. Ultraviolet mutagenesis and inducible DNA repair in Escherichia coli. Bacteriol. Rev. 40 (1976) 869-907.
- Witkin E.M., Wermundsen I.E. (1979) Targeted and untargeted mutagenesis by various inducers of SOS function in Escherichia coli. Gold Spring Harbor symp. Quant. Biol. 43 (1976) 881-886.
- Witkin E.M. Ultraviolet mutagenesis and the SOS response in Escherichia coli. A personal perspective. Envorinmental and Molecular Mutagenesis, 14, sup. 6 (1989) 13-34.
- Polard E.C., Person S., Rader M. And Fluke D.J. Relation of ultraviolet light mutagenesis to a radiation-damage inducible system in Escherichia coli. Radiation Research 72 (1989) 519-532.
- Mount D.W. and Kosel C. Ultraviolet light-induced mutation in UV-resistant, thermosensitive derivatives of lex A-srain of Escherichia coli K-12. Molc. Gen. Genet. 136 (1975) 95-106.
- Donnelly C.E. and Walker G. (1989) groE mutations of Escherichia coli are defective in umu DC dependent UV mutagenesis. Journal of Bacteriology 11 (1975) 6117-6125.
- Dutreix M., Moreau P.L., Bailone A., Gilert F., Battista J.R., Walker C. and Devoret R. (1989). New recA mutations that dissociate the various recA protein activities in Escherichia coli provide evidence for additional role for recA protein in UV mutagenesis. Journal of Bacteriology. 5 (1989) 2415-2423.
- Witkin E.M., McCall O.J., Volkert M.R. and Wermundse L.E. Constitutive expression of SOS functions and modulation of mutagenesis resulting from resolution of genetic instability at or near the recA locus of Escherichia coli. Mol.Gen.Genet., 185 (1982) 43-50.
- Defais M., Fauquet P., Rodman M. And Errera M. Ultraviolet reactivation and ultraviolet mutagenesis of l -phages in different genetic systems. Virology. 43 (1971) 495-503.
- Kochevar E.I. and Buckly L.A. Photochemistry of DNA using 193 nm excimer laser radiation. Photochem. Photobiol. 51 (1971) 527-532.
- Gurzadyan G.G., Ispiryan R.K. and Voskanyan K.Sh. Two-quantum photoprocesses in DNA under picosecond laser UV irradiation at 216 and 270 nm. Photochem. Photobiol. B: 11 (1991) 269-275.
- Voskanyan K.Sh. 633 nm light induces mutations. Studia Biophysica, v.139, 1 (1990) 43-46.
- Xiang Yang A study of the mutagenic effect of such physical factors as laser on E.coli and of the auxotrophic analysis. Proc. Inc. Conf. On Lasers in the Life Sciences, June 20-23 (1990) China.
- Avakyan Ts.M., Voskanyan K.Sh., Simonyan N.V. About some similarities of ionizing and laser radiation on bacteria cells. Reports of Armenian Academy of Sciences. 1 (1988) 32-35.
- Arutyunyan A.H., Avakyan Ts.M., Voskanyan K.Sh., Simonyan N.V. Efficiency of laser radiation action on bacterial cells depending on irradiation power and dose. Studia Biophysica. 128 (1988) 21-25.
- Tiphlova O. And Karu T. Action of low-intensity laser radiation on Escherichia coli. Critical Reviews in Biomedical Engineering. 18, Issue 6 (1991) 387-411.
- Karu T. Photobiology of low-power laser effects. Health Physics. 56 (1989) 692-704.
- Ohshiro T. and Calderhead R.G. Low level laser therapy. A practical introduction. John Wily & Sons Chichester (Ed.) 1988.
- Gamaleya N.F. Laser biomedical research in the USSR, in Laser Application in Medicine and Biology v.3, Wolbarsht M.L., (Ed.) Plenum , New-York, 1977.
- Pimenova M.N., Grichyshkina N.N., Asova L.G., Semenova E.V., Melnikova S.I. Practice of Microbiology, Prod. Moscow University, (1983) 137-139.
- Tokareva B., Amirtaev K.G., Krasavin E.A., Kozubek S. Express of Lac- mutations in E.coli bacteria by the "sandwich seeded" method. JINR, Dubna, Russia, (1987) P19-87-1987.
- Bresller S.E. The mechanism and the kinetics of reparation mutagenesis. Genetika, 12 (1976) 153-160.
- Harm W., Stein W. Zur dentung von maxima and sattigungs effecten bei dosis-effect-kurven fur strahleninduzierte mutation. Naturforchung 115 , (1956) 85-105.
- Myasnik M.N. The genetic control of bacteria sensitivity. Atomizdat, Moscow, 1974.
- Howard-Flanders P., Simson E., Hteriot L. The excision of thymine dimers from DNA filament formation and sensitivity to ultraviolet in E.coli K-12 Mutat. Res., 1, 3, (1974) 219-225.
- Boreyko A.V., Krasavin E.A. Mutagenic action of radiation with different LET on Bacillus Subtilis cells. Radiobiologia, Radioekologia ,7, 3 (1997), 408-412.
- Gurzadyan G.G. and Ispiryan R.K. (1990) Efficiency of laser photolysis of nucleic acids at 216 nm. Proc. Inc. Conf. On Lasers in the Life Sciences, June 20-23, China.