| Papers and Posters | Site Home Page |
Could irradiation with monochromatic light of visible spectral region cause genetic effects?
T.I.Karu, L.E.Bakeeva, V.M. Manteifel
Laser Technology Research Center of the Russian Academy of Sciences, 142092 Troitsk, Moscow region, Russian Federation
A.N. Belozersky Institute of Physico-chemical Biology, Moscow Lomonosov State University, 119899 Moscow, Russian Federation
Corresponding author
Abstract
The structure of mitochondria of yeast cells Torulopsis sphaerica was studied in cells of 6th -7th generations after irradiation of initial culture with a He-Ne laser (632.8 nm; 460J/m2 or 1150 J/m2). Ultrathin sections of cells were studied by electron microscopy (morphometric analysis of random sections and spatial reconstruction of chondriome by serial sections). The irradiation caused ultrastructural changes in chondriome of cells-descendants, which were in agreement with the level of functional activity of mitochondria. It is supposed that the irradiation with He-Ne laser regulates synthesis of mitochondrial enzymes on genetic level.
Introduction
It is generally accepted that mitochondria are sensitive to irradiation with various wavelengths of visible light. Activation of oxygen consumption, changes in electrochemical, biochemical, and optical properties of isolated mitochondria under illumination are well documented (reviews [1-3] and references therein).
All experiments mentioned above were performed with directly irradiated cells or mitochondria. On the other side, the proliferation of mammalian cells cultures (review [4]) as well as the division of yeasts [5,6] were increased during rather long periods after the irradiation. Some respiratory chain enzymes were activated in next generations of irradiated yeast cells [7].
It is known that the irradiation with a He-Ne laser caused an increase in frequency of chromosome aberrations in diploid cells of human fibroblasts [8] and irradiation with a semiconductor laser at 660 nm increased output of single-strand breaks of DNA in dose-dependent manner [9].
The long-term effects of He-Ne laser irradiation on proliferation of cells [4-6] and activity of respiratory enzymes in successive generations [7] as well affection of DNA [ 8,9] can not be explained by a direct action of visible light on DNA. The radiation with l =632.8 nm or l =660 nm can not cause mutations through direct action upon DNA. The energy of these photons is too low (~ 1.7 eV) to cause ruptures of covalent bonds in a molecule. DNA and RNA also do not have absorption bands in the visible spectral region. One can suppose that mutations can be caused via secondary messengers. The reactive oxygen species (ROS) could be considered in this role. It is known that some ROS like superoxide anion O2 - and the product of its dismutation H2O2 are generated in normal mitochondrial activity. It was supposed that the activation of respiratory chain by irradiation insreases output of these ROS (reviews [2,3]. One can not exclude a possibility that the oxidative stress is mutational primarily for the mitochondrial DNA and may be also for nuclear DNA.
As a first step in a series of studies of possible mutational action of monochromatic ned light we investigated the ultrastructure of the mitochondria of successive generations of initially irradiated with a He-Ne laser cells.
Material and methods
The experiments were performed with yeast culture Torulopsis sphaerica. Cells were cultivated as described in [ 5] and irradiated in the phosphate buffer with He-Ne laser in doses 460 J/m2 or 1150 J/m2. These two doses were chosen inasmuch as the protein synthesis and activity of two respiratory chain enzymes (NADH-dehydrogenase and cytochrome c oxidase) were significantly elevated above the control level in these two points of dose dependencies [ 7] . Method of the irradiation was the same as described in [ 7] . After the irradiation in the phosphate buffer the yeast was replaced into liquid nutrient (Reder medium with 1-% glucose) and grown with intense aeration for 18h (to 6th- 7th successive generations of initially irradiated cells), then harvested, fixed with KmnO4 and OsO4, dehydrated with ethanol solutions, and embedded into Epon 812 (detailed description of these procedures can be found in [ 10] ). Ultrathin sections were cut using an LKB III ultratome, contrasted with acetate and lead citrate, examined and photographed in HU-12 (Hitachi) electron microscope. Two methods of investigation were used: morphometry of random sections and spatial reconstruction by serial sections.
Results and discussion
According to the electron microscopy, the structure of T. sphaerica mitochondria was found to be similar to that of yeast cells with active respiration and oxidative phosphorylation [11]. The internal mitochondrial membrane formed cristae and was closely aligned with the external membrane. The intermembrane gap and intracristae space had a constant width (~30 nm).
In control cultures as well as in cultures irradiated at 460 J/m2, the mitochondrial profiles were round or oval, sometimes elongated. No elongated mitochondria were found in cultures initially irradiated at 1150 J/m2. The detailed morphological description and analysis of our experimental material is published elsewhere [10,12]. As a whole, electron microscopy did not reveal any changes in mitochondria that might be considered degenerative: impairment of outer and inner mitochondrial membranes, swelling or the matrix contraction.
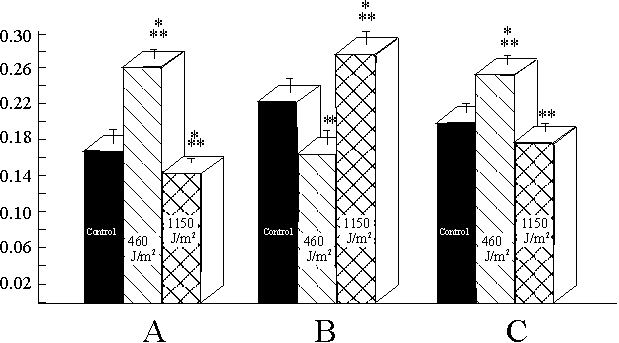
The following conclusions can be drawn from the results of morphometric analysis. First, the average area of a mitochondrion increased significantly only in cultures irradiated at 460 J/m2 (Fig. 1a). Second, the number of mitochondria per m m2 of cell cytoplasm decreased significantly in the cultures irradiated at 460 J/m2 and increased in the cultures irradiated at 1150 J/m2 (Fig. 1b). Third, the relation of the area occupied by cristae to the mitochondrial area significantly increased in the cultures irradiated at 460 J/m2 and decreased in the cultures, irradiated at 1150 J/m2 (Fig. 1c).
|
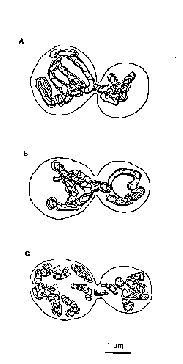
Spatial reconstruction of the mitochondrial apparatus of intact budding cells established presence of one branched giant mitochondrion and in average three mitochondria organized in the reticulum. As seen in Fig. 2a, a part of the giant mitochondrion is displaced into the bud.
The reconstruction of the chondriome of the budding cells which ancestors were irradiated at 460 J/m2 evidenced the presence of one giant mitochondrion. The giant mitochondrion was distributed between the mother cell and the bud (Fig. 2b). the chondriome also involved ordinary small organelles.
Cells which ancestors were irradiated in dose 1150 J/m2, were characterized by changing of spatial organization of the chondriome as compared with both control cells and the cells from the another irradiated group. A great number of small organelles was revealed in the budding yeast cells (Fig. 2c). giant mitochondria were not found in this experimental group.
|
In our study we found changes in the ultrastructure of the chondriome of yeast cells which ancestors were irradiated with a He-Ne laser. In both irradiated groups, the ultrastructure of mitochondria was found to be different from that of the control cells.The chondriome of the cells which ancestors were irradiated at the dose 460 J/m2 was found to be different from that of control cells only quantitavely (Figs. 1,2). In case of the irradiation at the dose 1150 J/m2, the mitochondrial apparatus of cells-descendants differed from that of control cells both quantitavely (Fig. 1) and qualitatively (Fig. 2).
The presence of giant mitochondria in yeast cells is not an unique phenomenon. For example, this type of mitochondrial apparatus was found in yeasts during their switching from aerobic respiration to glycolysis [ 13] , or in cultures grown with limited glucose [ 14] . At the same time, the giant mitochondria were also present in exponentially growing yeasts with active respiration [ 15] . Those examples evidence an ambiguous functional role of giant mitochondria. Inasmush as all yeast cultures in our experiments were in the same (exponental) phase of growth and were also cultivated in the same conditions in presence of glucose and oxygen, one can not connect changes in chondriome found in our experiments with variations in life cycle of yeasts and cultivation conditions.
One can find an accordance between the morphological changes of condriome in irradiated cells (Fig. 1) and activity of respiratory enzymes in the same cultures [ 7] . The mitochondria of cells which ancestors were irradiated at 460 J/m2 were characterized by increased area of cristae and increased activity of NADH-dehydrogenase and cytochrome c oxidase. A correlation between increase in the area of mitochondrial cristae and activity of respiratory enzymes [16-18] as well as between decrease of area of cristae and inhibition of respiration [19] and decrease of transmembrance potential [20] has been described. A fragmentation of mitochondria per se was found by treating the cells with inhibitors of respiratory chain [21,22]. The area of cristae per area of a mitochondrion was decreased back to the control level in cells of the culture initially irradiated at 1150 J/m2. Also, the activity of the respiratory enzymes was decreased as compared with respective data for the cells initially irradiated at 460 J/m2 [ 7] . It is known that a decrease in the area of the cristae and an increase in the mitochondrial number per cell section were correlated with a decrease of respiration and oxidative phosphorylation [ 23,24] .
Spatial reconstruction of the chondriome evidenced that in two cases (control group and the culture initially irradiated at 460 J/m2) the giant mitochondria were present but they are absent in the culture intially irradiated at 1150 J/m2 (Fig. 1). Recall that the spatial reconstruction was carried out in all cases in budding cells after nucleokinesis when the cytokinesis was not finished. It means that the established changes in the structure of chondriome were not connected with being the cells at different points of the cell cycle. It is known that during cell cycle mitochondria are fragmenting to discrete organelles or fusioning into giant structures [ 25,26] .
So, both morphometric and spatial analyses of the condriome of cells-descentants of irradiated cultures testified that there are changes in the ultrastructure of the mitochondrial apparatus. These changes depended on the irradiation dose and correlated with functional activity of the respiratory chain. Inasmuch as the experiments were performed with cells of 6th-7th generations of initially irradiated cultures, one can suppose that these changes are of genetic origin.
Mitochondrial ATP synthesis (oxidative phosphorylation) is regulated by the membrane potential (respiratory control) and protein synthesis (transcription control) (reviews [27, 28]). First type of regulation can occur and really occurs only in directly irradiated cells (reviews [1-3]), so in the present case one can speak mainly about possible transcription control. Following experimental finding supports the suggestion about involvement of transcription control. Both mitochondrial and nuclear DNA encode four enzyme complexes of oxidative phosphorylation. These are F0F1, complex I, complex III and complex IV [27]. Recall here that the activity of NADH-dehydrogenase and cytochrome c oxidase [ 7] belonging respectively to complexes I and IV, was increased.
We found that the total area of chondriome per area of cytoplasm was practically not changing due to the irradiation [ 10] . This fact could point to the absence of the activation of replication of mitochondrial DNA (mtDNA) [ 16,29] . It means that the changes in the ultrastructure of the inner mitochondrial membranes (cristae) correlating with the changes in the activity of two enzymes of respiratory chain [ 7] could be due to some changes in the transcription and/or translation functions of mtDNA.
It is known that mtDNA is particularly sensitive to oxidative damage and reactive oxygen species (ROS) can cause mutations in mitochondrial DNA rather easily (review [30]). It was proposed that increased production of ROS like superoxide anion (and the product of the dismutation, H2O2) as a result of direct light activation of respiratory enzymes can be considered among primary mechanisms when monochromatic visible light affects mitochondria (reviews [2, 3]). It means that one can not exclude a possibility of mutational action of these ROS in directly irradiated cells. The result of this action can appear in the cells-descendants.
The results of the present paper demonstrate that cells-descendants of the initially irradiated with a He-Ne laser culture have quantitative or qualitative (depending of the dose) changes in the mitochondrial ultrastructure. These results give grounds to suppose that the irradiation with He-Ne laser causes not only rapid regulation of ATP synthesis in directly irradiated cells (reviews [1-3] ), but also can affect the control of mitochondrial activity via protein synthesis (transcription and/or translation control) which is probably realized on genetic level.
*The full-length paper "Long-term effects of He-Ne laser radiation: changes in ultrastructure of chondriome in successive generations of yeast cells Torulopsis sphaerica" by V.M.Manteifel, L.E.Bakeeva and T.I.Karu will be submitted to J. Photochem. Photobiol., B:Biol. in June 1999.
References [ 1] C.Salet, S.Passarella, E.Quagliariello, Effects of selective irradiation on mammalian mitochondria, Photochem. Photobiol. 45(1987) 433-438. [ 2] T.Karu, Primary and secondary mechanisms of action of visible to near-IR radiation on cells, J. Photochem. Photobiol. B: Biology 49 (1999) 1-17. [ 3] T.Karu, The Science of Low Power Laser Therapy, Gordon and Breach Sci. Publ., London, 1998. [ 4] T.Karu, Effects of visible (laser) radiation on cultured cells, Photochem. Photobiol. 52 (1996) 1089-1099. [ 5] G.E.Fedoseyeva, T.I.Karu, T.S.Lyapunova, N.A.Pomoshnikova, M.N.Meissel, The activation of yeast metabolism with He-Ne laser radiation. I. Protein synthesis in various cultures, Lasers Life Sci. 2 (1988) 137-146. [ 6] G.E.Fedoseyeva, T.I.Karu, V.S.Letokhov, V.V.Lobko, N.A.Pomoshnikova, T.S.Lyapunova, M.N.Meissel, Effect of the He-Ne laser radiation on the reproduction rate and protein synthesis of the yeasts, Laser Chemistry 5 (1984) 27-33. [ 7] G.E.Fedoseyeva, T.I.Karu, T.S.Lyapunova, N.A.Pomoshnikova, M.N.Meissel, The activation of yeast metabolism with He-Ne laser radiation. II. Activity of enzymes of oxidative and phosphorous metabolism, Lasers Life Sci. 2 (1988) 147-154. [ 8] B.I.Stepanov, V.A.Mostovnikov, A.N.Rubinov, I.V.Khokhlov, The regulation of functional activity of human cells by laser irradiation, Doklady Akademii Nauk SSSR, 236 (1977) 1007-1009. [ 9] V.J.McKelevey, A.L.Keegan, J.A.Allen, Induction of DNA damage by low level laser irradiation in Friend mouse erythroleukemia cells, Mutation Res. 271 (1992) 131. [ 10] V.M. Manteifel, V.I.Biryusova, N.A.Kostrikina, T.I.Karu, Morphometric study of the changes in yeast Torulopsis sphaerica chondriome induced with He-Ne laser irradiation, Molecular Biology (Moscow), 30, Part II (1996) 834-839. [ 11] V.I.Biryusova, Ultrastructural Organisation of Yeast Cells, Nauka, Moscow, 1993 (in Russian). [ 12] L.E.Bakeeva, V.M.Manteifel, T.I.Karu, Action of He-Ne laser radiation upon ultrastructure of mitochondrial apparatus of next generations of yeast cells Torulopsis sphaerica, Cytology (St.Petersburg), 1999 (in press). [ 13] N.M.Meissel, V.I.Biryusova, T.M.Volkova, M.G.Malyagin, Functional morphology and cytochemistry of mitochondrial apparatus of microorganisms. In: Electronic and Fluorescent Microscopy of Cell. Nauka, Moscow, 1964, pp.1-15 (in Russian). [ 14] W.Visser, E.A.van Spronsen, N.Nanninga, J.T.pronk, J.Gijs Kuenen, J.P.van Dijken, Effect of growth conditions on mitochondrial morphology in Saccharomyces cerevisiae, Ant. Van Leewenhoek, 67 (1995) 243-253. [ 15] B.Stevens, Mitochondrial structure. In: The Molecular Biology of the Yeast Saccharomyces. Life Cycle and Inheritance (Strathern J.N., Jones E.W., Broach J.R., eds.) Cold Spring Harbor Laboratory, N.Y. 1981, pp. 471-504. [ 16] F.Djouadi, J.Bastin, T.Gilbert, A.Rotig, P.Rustin, C.Merlet-Benichou, Mitochondrial biogenesis and development of respiratory chain enzymes in kidney cells: role of glucocorticoids, Am. J. Physiol. 267 (1994) C245-C254. [ 17] C.G.Vellejo, M.Lopez, P.Ochoa, M.Manzanares, R.Garesse, mitochondrial differentiation during the early development of the brine shrimp Artemia tranciscana, Biochem. J. 314 (1996) 505-510. [ 18] C.D.Moyes, O.A.Mathieu-Castello, N.Tsushiya, C.Filburn, R.G.Hansford, Mitochondrial biogenesis during cell differentiation, Am.J.Physiol. 272 (1997) 1345-1351. [ 19] M.A.Hatab, P.A., P.A.Whittaker, Isolating and characterization of respiration-deficient mutants from pathogenic yeast Candida albicans. Ant. Van Leewenhoek 61 (1992) 207-219. [ 20] E.Vega Nunez, A.M.Alvarez, A.Menendez-Hurtado, A.Santos, A.Perez-Castillo, Neuronal mitochondrial morphology and transmembrane potential are severely altered by hypothyroidism during brain development, Endocrinology, 138 (1997) 3771-3778. [ 21] L.V.Johnson, M.L.Walsh, B.Boscus, L.B.Chen, Monitoring of relative mitochondrial membrane potential in living cells by fluorescence microscopy, J. Cell Biol. 88(1981), 526-535. [ 22] L.V.Johnson, M.L.Walsh, L.B.Chen, Localization of mitochondria in living cell with Rhodamine 123. Proc. Natl. Acad. Sci. USA 77(1980) 990-994. [ 23] Z.Baranowski, B.Hrebendo, M.Cieslawska, Division of Physarum mitochondria during starvation, Cell Biol. Int. Rep. 15 (1991) 197-204. [ 24] A.Markowska, P.Robuflat, G.Gottardo, G.Mazzochi, G.G.Nussdofer, Age-dependent changes in the function and morphology of rat adrenal zone fisiculta, Histol.-Histopathol. 9 (1994) 263-268. [ 25] K.Tanaka, T.Kanbe, T.Kuroiwa, Three-dimensional behavior of mitochondria during cell division and germ tube formation in the dimorphic yeast Candida albicans, J. Cell Sci. 73 (1985) 207-220. [ 26] T. Kanbe, J.Kabayashi, K.Tanaka, Dynamics of cytoplasmic organelles in the cell cycle of the fission yeast Schizosaccharomyces pombe: three-dimensional reconstruction’s from serial sections, J. Cell Sci. 94(1989) 647-656. [ 27] A. Tzagaloff, A.M.Myers, Genetics of mitochondrial biogenesis, Annu. Rev. Biochem. 55 (1986) 249-185. [ 28] Y.Kagawa, S.Ohta, Regulation of mitochondrial ATP synthesis in mammalian cells by transcriptional control, Int. J. Biochem. 22 (1996) 219-229. [ 29] K.L.Black, T.Shiraishi, K.Ikezak, K.Tabuchi, D.P.Becker, Changes of mitochondrial mass in the hemopoietic stem cell line FDCP-Mix after treatment with etoposide: a corrective study by multiparameter flow cytometry and confocal and electron microscopy, Exp. Cell Res. 221 (1995) 281-288. [ 30] T.Ozawa, Genetic and functional changes in mitochondria associated with aging, Physiol. Rev. 77 (1997) 425-464. |