| Papers and Posters | Site Home Page |
Membrane and soluble fractions of adenylyl cyclase from Sorghum bicolor seedlings positively react to the action of red and far red lights.
Kerim G. Gasumov, Chizuko Shichijo*, Shahniyar M. Bayramov, Tohru Hashimoto*
Institute of Botany, Patamdar Shosse 40, Baku 370073, Azerbaijan, *Kobe University, Nada-ku, Kobe 657, Japan
Abstract
The existence of membrane and soluble fractions of light induced adenylyl cyclase from Sorghum bicolor seedlings have been demonstrated. These fractions have shown difference in reaction to red and far red light effects. Membrane fraction has a seasonal modification in regard of reaction to red and far red lights. Autumn far red light induced form of membrane-bound adenylyl cyclase in spring is substituted by red light sensible form. But soluble fraction of adenylyl cyclase sensible for both red and far red lights and dos not change sensibility from dependence of season.
Introduction
Cyclic adenosine monophosphate (cAMP) is widely distributed from prokaryotes to eukaryotes as a signal molecule. In most of these organisms the presence of cAMP can be well-defined physiological processes. In animal cells, cAMP functions as a second messenger in cellular signal transduction processes. The increasing of cAMP level in cells promotes by the activation of protein kinase A phosphorilation of several intracellular enzymes and the phosphorilation results in increasing of their enzymatic activities. In contrast to the well-documented situation in the animal kingdom, the presence of cAMP in higher plants and its physiological role in plant signal transduction are quite obscure. Only in the last decade exciting new evidence has created new momentum in this field. The occurrence of 3-,5--cAMP in plants has been established (1, 2), moreover its synthesis by adenylyl cyclase in low plants is widely accepted (3-5) and existence of adenylyl cyclase activity in higher plants is persistently growing. Adenylyl cyclase was shown to be present in Phaseolus vulgaris, Medicago sativa, Risinus communis, Pisum sativum by physiological and biochemical experiments (6-9). Light regulated adenylyl cyclase have been shown to be present in Zea maiz and Sorghum bicolor (10,12) and genes of light regulated adenylyl cyclase were isolated and characterised from cyanobacteria (4).
One of physiological effect of 3-,5--cAMP participation low and higher plant signal transduction processes is actively studied. It was shown that red and far red lights absorbed by phytochrome regulate activity of cAMP related enzymes in maize seedlings and red illumination of etiolated seedlings results to increasing of cAMP level in cell (10,11). In a nitrogen-fixing cyanobacterium Anabaena cylindirica, an immediate light-to-dark transition caused a 9-fold increase in cAMP concentration within 1 min. (13) and these authors have shown existence of light regulated membrane and soluble fraction of adenylyl cyclase in Spirulina platensus (5).
Here we report about existence of membrane and soluble forms of light regulated adenylyl cyclase in sorgum plants. Both of fractions we are considering to participate in plant photosygnal transduction process synthesising 3-,5--cAMP from ATP.
Methods
In experiments 5 days etiolated seedlings of Sorghum bicolor as a plant material was used. Seeds of Sorghum bicolor Moench, cv. Acme Broomcorn, were soaked for 24 h in a water bath of 24oC, in which temperature-adjusted tap water being supplemented circulated. Seedlings were grown for 5 days at 20oC. The duration and temperature of growing were selected for maximum anthocyanin synthesis (14,15).
Light source and irradiation of seedlings
As a red light source RLED lmax = 670 nm, (half-bendwidth 655-685 nm) red light emitting diodes, (QB-1310CS-670-735; Quantum Revices, Inc., Wisconsin, USA) and far red light source FRLED l max-750 (half-bendwidth 730-760nm), far red light emitting diodes (QB-1310CS-735, Quentium Revices, Inc., Wisconsin, USA) were used.
Etiolated seedlings were irradiated according to Shichijo et al. (1993) by R in the intensity of 100 mmol m-2 s-1 x 200 s (20000 mmol m-2) and FR 400 mmol m-2 s-1 x 30 s (12000 mmol m-2) where intensity of R is plateau for anthocyanin synthesis (14). All processes of irradiation were carried out under green safelight.
Adenylyl cyclase preparation.
Adenylyl cyclase protein samples were prepared from etiolated seedlings. Homogenisation of seedlings conducted by glass mortar. For 1 g seedlings used 3ml Tris-HCl buffer pH 7.4 containing: 25 mM Tris (hydroxymethyl)-amminometane, 5 mM MgCl2, 0,25 mM NaEDTA and 1mM Dithiothreitol (threo-1,4 Dimercapto-2,3-butanediol).
Homogenisation of seedlings was conducted in ice bath (+2 - 4oC), homogenate filtered through four layer cheesecloth. The filtered homogenate was subjected to centrifugation at 1000g for 10 min, and the pellet discarded asunhomogenised culture and cell fragments (Fig 1). Supernatant was recentrifuged at 30,000g for 40min +2-3oC. Pellet (membrane fraction, enrich with adenylyl cyclase activity) dissolved in 2 ml extraction buffer and was dialysed against 4L the same buffer during 18-20 hr.
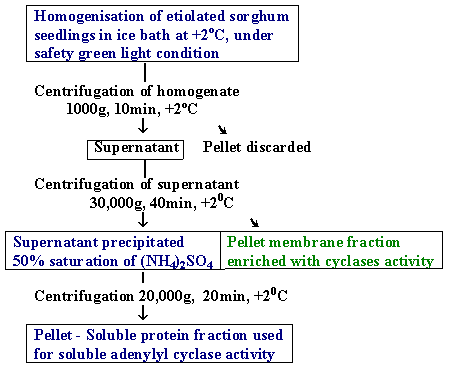
Figure 1. Fractionation and preparation of membrane and soluble fractions of adenylyl cyclase samples from Sorghum seedlings. Process of isolation was carried out in +2-4oC, under safety green light condition. After isolation protein samples were dialised against 4 L Tris-HCl buffer (Tris 0.01M, pH-7.4, MgCl4 2mM, b-mercaptoethanol) during 18-20 hr.
Supernatand subjected for isolation of soluble fraction of adenylyl cyclase activity. Soluble fraction was separated by precipitation of (NH4)2SO4 in a 50% of saturation of (NH4)2SO4, and stirred 60 minute at +3oC and was subjected centrifugation at 20000g for 20 min Pellet dissolved in 2ml Tris-HCl buffer in composition: Tris 0,01M, pH 7.4, MgCl2 2mM, DTT 2mM. Obtained soluble protein fraction was dialysed against 4L Tris-HCl buffer (Tris 0.01M, pH-7.4, MgCl4 2mM, b-mercaptoethanol) for 18-20 hr.
Adenylyl cyclase activity assay.
Adenylyl cyclase activity was determined with some modification by (16). The standard assay was done in epindorf tubes in the 50 ml incubation mixture containing 40mM Tris-HCl, 5mM MgCl2, 0.25mM Na-EDTA, 0.5mM dithiothreitol, 20mM NaF, 5mM cafeine, 4mM phosphocreatine, 8 mg/ml creatine phosphokinase, 1mg/ml BSA, 0.2mM ATP, 14C-ATP 0.1mCi, protein 6-9mg. The incubation mixture was incubated at the 33oC for 20 min and incubation was terminated adding of 5ml of 20mm cAMP and boiling 2min, after boiling samples were cooled in ice bath. Then incubated tubes was centrifuged 5min 12000 RPM. Supernatant was used for separation of nucleotides. Separation of nucleotides were carried out on thin layer chromotography on silicagel produced by "Merck". Before spotting of samples on plate we spotted same point 2ml solution of "nucleotide witnesses" containing 10mM of each nucleotides - ATP, ADP, AMP, cAMP, adenosine and adenine and then on the spotted and dried witnesses we spotted 6ml incubation mixture. Chromotography was developed in the system of solution: n-butanol:acetone:ammonia (25%) in relation 8:2:7 and again the same system in relation 8:2:2. Nucleotides visualised by UV light, cut and counted in toluone sinntillation liquid containing in 1L toluone 0.2g Dimethyl-POPOP and 4g PPO (2,5-Diphenyloxasol) on the "Becman LS 6000" liquid scintillation spectrometer.
Protein concentrations were determined using method of Lowry.
Results and discussions
Annual maximum of ACse activity for both fractions differs.
Soluble and membrane fractions of adenylyl cyclase were isolated from etiolated seedlings. After homogenising of seedlings (Fig.1,) and discarding of non-homogenised cell and culture fragments, we fractionated object for membrane and soluble protein fractions precipitating by ammonium sulphate. Obtained membrane and soluble fractions were subjected for adenylyl cyclase activity assay. High cylase activity have been determined for both fractions and their reaction to the influence of monochromatic red and far red lights. Both fractions we have found to appear high cyclase activity in slow alkaline medium (pH-7.6 membrane fraction and 7.8 soluble fraction).
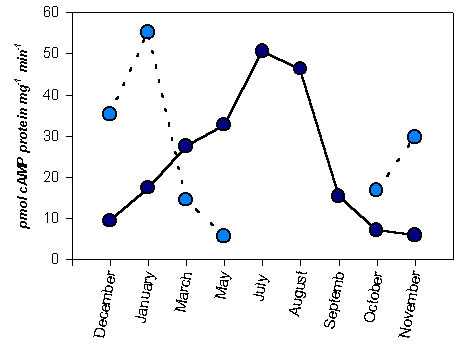
Figure 2. The variation of adenyl cyclase activity in dependence of season of the year. Enzyme activity was assayed on the membrane fraction of 5 days etiolated Sorghum bicolor seedlings grown at 20oC. ¾ ¡ ¾ activity of membrane fraction of denylyl cyclase; - - ¡ - - activity of soluble fraction of adenylyl cuclase Enzyme activity assay was done in epindorf tube in the 50 ml mixture containing 40 mM tris-HCl, 5mM MgCl2, 0.25mM Na-EDTA, 0.5mM DTT, 20mM NaF, 5mM cafeine, 4mM phosphocreatine, 8 mg/ml creatine phosphokinase, 0.2mM ATP, 14C-ATP 0.1mCi, in sample, protein 6-9mg. Incubation was conducted at the 33oC for 20 min and was terminated by adding of 5ml 20mM cAMP boiling for 2 min.
fraction of adenylyl cyclase appears maximum enzyme activity in spring and summer time. Obviously it is associated with starting of physiologically active life of plants in this season. Activity of this fraction is sharply fallen in late of summer and autumn, although (Fig.2,) low enzyme activity was appeared and in this period. On the contrary, the soluble fraction of adenylyl cyclase we have found to appear higher enzyme activity in early and middle of winter. There exist evidence that adenylyl cyclase activity from phycomyces at 100000g centrifuged in supernatant higher than in pellet (16). In our experiments the total enzyme activity of soluble fraction significantly higher than activity of membrane fraction. But with coming of spring the enzyme activity of this fraction have been found to be decreased and it disappeared fully in summer and autumn period (Fig.2). It was again appeared only at the late of autumn.
Seasonal difference in light effects on membrane bound adenylyl cyclase.
To investigate whether adenylyl cyclase activity can be regulated by phytochromes we subjected the etiolated seedlings for irradiation by monochromatic red and far red lights before homogenisation. The seedlings just after irradiation were homogenised and separated to membrane and soluble fractions, and both fractions conducted for adenylyl cyclase activity assay.
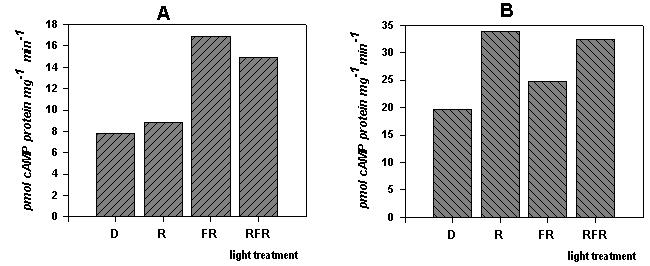
Figure 3. Seasonal alteration of the reaction of adenylyl cyclase on the action of red and far red lights.
Etiolated Sorghum bicolor were irradiated by red (100 mmol m-2 s-1 x 200s = 20000 mmol m-2) and far red (400 mmol m-2 s-1 x 30s = 12000 mmol m-2) lights. A - data for experiments conducted in autumn (Sept.-Oct.); B - date for experiments conducted in spring (March-May). R - seedlings irradiated by red light; FR - seedlings irradiated by far red lights; RFR - seedlings irradiated by far red light immediately after red irradiation intensity of light as above; D- enzyme fraction isolated from non-irradiated etiolated seedlings;
Figure 3 shows seasonal difference in reaction of adenylyl cyclase to the action of irradiation of seedlings. In autumn red light did not exert influence on adenylyl cyclase activity, while significantly increasing of activity we observed at the irradiation of seedlings by far red light and far red after red irradiation. On the contrary in spring we observed remarkable activation of adenylyl cyclase by red light while effect of far red was negligible. Moreover irradiation of seedlings by far red after red light did not reverse its activating effect fully.
From these data we can suppose in autumn either exist only phytochrome A which is activated by far red lights and active phytochrome A increase adenylyl cyclase activity, or there is a form of adenylyl cyclase which react only on the action of phytochrome A. If consider in autumn red light increase guanylyl cyclase activity (12) and soluble adenylyl cyclase (Figure 4) we can conclude existence of different forms of adenylyl cyclase which are appeared in different season of year. Form of adenylyl cyclase is displayed in autumn sensible only to the action of phytochrome A, and another form which is appeared in spring and early summer sensible to the action of phytochrome B. It seems like in spring activated by red light phytochrome B causes a certain steady modification of adenylyl cyclase molecule which does not reverse by far red light.
Soluble adenylyl cyclase more sensible to red and far red lights than membrane fraction.
As shown above irradiation of seedlings by red and far red lights led to alteration of membrane fraction of adenylyl cuclase activity and it produces great interest that, effects of these lights differ in autumn and spring, we explored soluble adenylyl cyclase reaction in response of these lights action.
As illustrated in Figure 4, soluble adenylyl cyclase unlike membrane fraction is more sensible for all used lights. In spring where the membrane bound adenylyl cyclase is significantly activated only by far red light, the soluble fraction positively reacts on the action of red and far red lights,
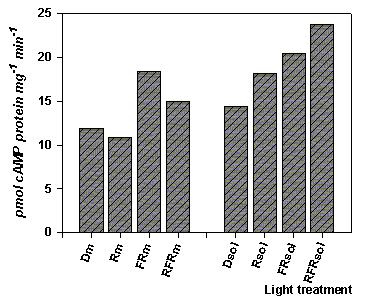
Figure 4. Red-far red light effects on soluble and membrane adenylyl cyclase activity. Data for experiments conducted late of autumn and early winter (Nov. Dec.).
R - irradiation of seedlings by red lights; FR - irradiation of seedlings by far red lights; RFR - irradiation of seedlings by red light following by red-far red lights; m - membrane fractions of protein samples; sol - soluble fraction of protein samples precipitated by 50% of saturation of sodium sulphate. Irradiation of seedlings and preparation of enzyme samples were conducted under green safelight condition and +2-3oC.
moreover effects of these lights summarised at dichromatic irradiation of seedlings by red-far red lights. The same light effects on soluble fraction we can also see in spring and early summer experiments (Figure 5). In this period membrane bound adenylyl cyclase reacts only to action of far red light, but soluble adenylyl cyclase is activated by all used lights. Furthermore the positive action of lights in early summer are appeared moor strongly, although in this period total enzyme activity of soluble adenylyl cyclase isolated from non-irradiated seedlings is lower.
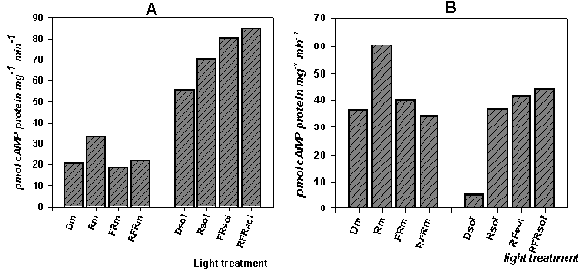
Figire 5. The action of irradiation of seedlings by red and far recd lights on adenylytl cyclase activity.
A - data for late of winter - early spring experiments (Feb. - March); B - data for early summer (May-June)
It is obviously, the same reaction of soluble adenylyl cyclase in different season of year testify that in sorghum plants exist only one form of soluble enzyme, and this adenylyl cyclase sensible to both of phytochromes A and B.
The obtained results most worth in that, multiple forms of adenylyl cyclase are being activated by monochromatic red and far red lights and it seems to be involved in the reception of light signals. Obviously adenylyl cyclase is not directly effected by light, more true approach to problem is first messanger of light signals - phytochrome. Activated by red-far red lights phytochrome may be phosphorylates and cause certain molecular modification of adenylyl cyclase which results its activation.
Taking into consideration of hypothesis on localisation of soluble enzymes in subcellular structure (17) we can suppose membrane and soluble forms of adenylyl cyclase localised in certain cell substructure. Reacting on the action of red and far red lights adenylyl cyclase can results local increasing of 3',5'-cAMP concentration. And this should be results activation of different cAMP-binding proteins which participate in the regulation of expression of plant genomes (18).
However there are still interesting question to be answered concerning the role of cAMP in particular light regulated adenylyl cyclase in plant growth regulation and photomorphogenesis. Evidence that light regulates adenylyl cyclases, but mechanism of this regulation requires further investigation and this investigation should be more molecular level. It is possible to manipulate experimentally, purifying both of adenylyl cyclases studying their structural organisation, and alteration under phytochrome absorbed lights.
REFERENCES
- Newton, R. P. and Brown, E. G. (1986) The biochemistry and physiology of cyclic AMP in higher plants. In Hormones, Receptors and Cellular Interactions in Plants (Chadwick, C. M., and Carrod, D. R. Eds.), pp.115-153, cambridge Univ. Press, Cambridge, UK.
- Newton, R. P., Gibbs, N., Moyse, C.D., Wiebers, J. C., and Brown E. G. (1980) Mass spectrometric identification of adenosine 3',5'-cylic monophosphate isolated from higher plant tissue. Phytochemistry, 19, 1909-1911.
- Franco, D. A., and Wetzwl, R. G. (1980) Cyclic AMP production and extracellular release from green and blue-green algae. Physiol.Plant. 49, 65-67.
- Katayama, M., Wada, Y., and Ohmori, M. (1995) Molecular cloning of cyanobacterial adenylate cycalse gene from the filamentous cyanobacterium Anabaena cylindrica. J. Bacteriol., 177, 3873-3878.
- Yashiro, K., sakamoto, T. and Ohmori, M. (1996) Molecular characterisation of adenylate cyclase gene of the cyanobacterium, Spirulina platensis. Plant Mol.Biol. 31,175-181
- Lusini, P., Trabalzini, L., Franchi, G.G., Bovalini, L., Martelli, P. (1991) Adenylate cyclase in roots of Ricinus communis stimulation by GTP and manganese. Phytochemistry 30(1), 109-111.
- Cooke, C. J., Smith, C. J., Walton, C. J., and Newton, R. P. (1994) Evidence that cyclic AMP is involved in the hypersensitive response of Medicago sativa to a fungal elicitor. Phytochemistry, 35(4), 889-895.
- Pacini, B., Petrigliano, A., Diffley, P., Paffetti, A., Brown, E. G., Martelli, P., Trabalzini, L., Bovalini, L., Lusini, P., and Newton R. P. (1993) Adenylyl cyclase activity in roots of Pisum Sativum. Phytochemistry, 34(4), 899-903.
- Roef, L., Witters, E., Gadeyne, J., Marcussen, J., Newton R. P., and Van Onckelen H. A. (1996) Analysis of 3',5'-cAMP and adenylyl cyclase activity in higher plants using polyclonal chicken egg yolk antibodies. Analt.Biochemistry, 233, 188-196.
- Fedenko, E.P., Ivanova, M. A., Doman, N. G., (1983) Adenylate cyclase and phytochrome. Dokl. Akad.of SSR (Reports of Academy of the USSR), 269(5), 1267-1269.
- Fedenko, E. P., Gasumov, K. K., Doman, N. G. (1988) Phosphodiesterase of cyclic AMP and phytochrome. Izvestiya Akad. SSR (News of Acad. of USSR) secsion biology, 5, 740-746.
- Gasumov, K. G., Shichijo, C., Hashimoto, T. (1997) Red - far red light effects on the adenylil cyclase and guanylil cyclase activity of sorghum bicolor seedlings. Proceedings 1st Internet Conf. on Photochem. and Photobiol., http://www.photobiology.com/v1/contrib.htm (C29)
- Ohmori, M. (1989) cAMP in Anabaena cylindrica: Rapid changes in cellular levels in response to changes in extracellular environments. Plant Cell Physiol., 30, 911-914.
- Shichijo, C., Hamada, T., Hiraoka, M., Johnson, C. B., Hashimoto, T. (1993) Enhancement of red-light-induced anthocyanin synthesis in sorghum first internodes by moderate low temperature given in the pre-irradiation culture period. Planta, 191, 238-245.
- Shichijo, C., Hamada, T., Johnson, C. B., Hashimoto, T. (1996) Effects of moderately low temperature (20oC) on phytochrome responces during preirradiation: anthocyanin synthesis in Sorghum bicolor at high- and low-Pfr/Ptot ratios. Photochem.and Photobiol., 63(3), 328-335.
- Cohen R. J., Ness J.L., Whiddon S.M. (1980) Adenylate cycalse from Phycomyces sporangiophore. Phytochemistry, 19, 1913-1918.
- Ryazanov, A.C. (1988) Organization of soluble enzyme in the cell: relay at the surface. FEBS Letters, 237 (1), 1-4.
- Yavorskaya, V.K., Kalinin, F. L., Dragovoz, I. V. (1987) Physiological role of cAMP-binding proteins in cells of rye seedlings. Fiziologiya Rasteniy (Plant Physiol.), 34(5), 963-969.