| Papers and Posters | Site Home Page |
Mycosporine-like amino acids in the marine dinoflagellate Gyrodinium dorsum: induction by ultraviolet irradiation
Manfred Klisch and Donat-P. Häder*
Institut für Botanik und Pharmazeutische Biologie, Friedrich-Alexander-Universität, Staudtstr. 5, D-91058 Erlangen, Germany
*Corresponding author: Tel.: +49 9131 8528216; fax: +49 9131 8528215
e-mail: dphaeder@biologie.uni-erlangen.de
Abstract
Cultures of the marine dinoflagellate Gyrodinim dorsum were exposed to
polychromatic radiation (PAR and UV) from a solar simulator for up to 72 h. Different
irradiance spectra in the ultraviolet were produced by inserting cut-off filters between
lamp and samples. The MAA content and composition were investigated by spectroscopic and
chromatographic analysis. The study revealed that G. dorsum contains a complex
mixture of several aminocyclohexenimine-MAAs and one aminocyclohexenone-MAA. UV
irradiation around 320 nm induced an increase in the concentration of all MAAs in the
samples. The presence of short wavelength UV-B radiation resulted in decreased overall MAA
production and a spectral shift in the absorption of the MAA mixture towards shorter
wavelengths as a result of altered MAA composition.
Keywords: mycosporine-like amino acids; phytoplankton; UV absorbing compounds;
ultraviolet radiation
Abbreviations: PAR, photosynthetically active radiation (400 - 700 nm); UV-A,
ultraviolet-A radiation (315 - 400 nm); UV-B, ultraviolet-B radiation (280 - 315 nm); MAA,
mycosporine-like amino acid; HPLC, high performance liquid chromatography; RT retention
time; Chl a, chlorophyll a
Introduction
The increased UV-B radiation reaching the Earth's surface due to the depletion of stratospheric ozone raised the question of the effect of this enhanced radiation on marine ecosystems. Like all phototrophic organisms phytoplankton are potentially subject to harmful effects of excessive UV-B radiation, such as DNA damage, inhibited photosynthesis and growth, and cell death in the end. In clearest oceanic waters UV-B radiation can penetrate several tens of meters and even in coastal waters that have a stronger attenuation of UV radiation, near the suface irradiances are present that can cause injurious effects to aquatic organisms [13]. Therefore phytoplankton have developed certain tolerance mechanisms to avoid harmful UV radiation. These include the vertical movement within the water column to avoid exposure to high doses of harmful radiation [1], repair of DNA damage [2] and the production of UV-absorbing compounds, MAAs [3-7].
The accumulation of MAAs is a widespread although not ubiquitous feature among phytoplankton species [6,8]. Most of the eukaryotic marine phytoplankton species that are known to accumulate MAAs are dinoflagellates and diatoms. In few species the effects of different spectral ranges on the formation of MAAs have been investigated and were found to differ considerably between species [17]. In most other studies the spectral resolution is restricted.
In the present study we have investigated the influence of different wavelength ranges
in the UV on the content and composition of MAAs in the marine dinoflagellate Gyrodinium
dorsum.
Materials and Methods
The organism
Gyrodinium dorsum originally isolated from Kattegatt was a gift from Dr. Ekelund
(Lund, Sweden). The organisms were grown in F/2 medium [10] that was
prepared using artificial sea water (Tropic Marine, Dr. Bienle GmbH, Germany). The culture
tubes were placed in a Kniese apparatus and bubbled with air. The culture temperature was
19° C and continuous illumination was provided by fluorescent tubes at an
irradiance of 35 W m-2 PAR. The cultures used in the experiments were in the
exponential phase of growth.
Irradiation treatment
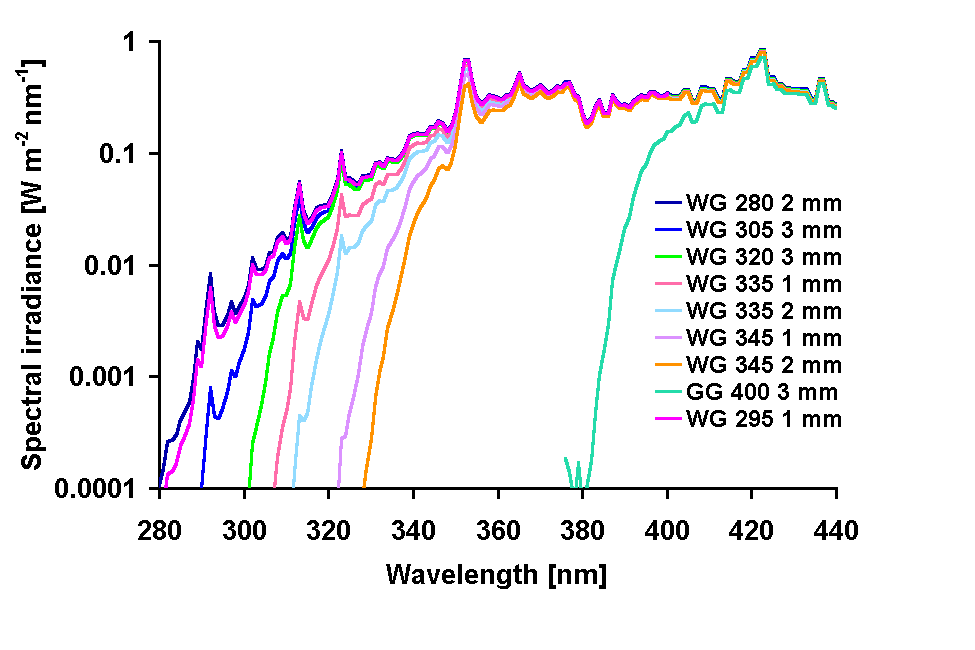
A solar lamp (Dr. Höhnle, Martinsried, Germany) was used to provide PAR and UV radiation. The organisms were exposed in open polystyrene containers covered with black adhesive foil from the outside to avoid stay light. The containers were covered by UV transparent acrylic and cut-off filters (Schott & Gen., Germany) of different cut-off wavelengths and thicknesses (GG 400, 3 mm, WG 345, 2 mm, WG 345, 1 mm, WG 335, 2 mm, WG 335, 1 mm, WG 320, 3 mm, WG 305, 3mm, WG 295, 1 mm, WG 280, 2 mm). The containers were placed in a water bath at 19 °C under the solar lamp at a distance of 119 cm. The irradiance of the light source was measured with a double monochromator spectroradiometer (OL 754, Optronic Laboratories, Orlando, FL, USA) and the irradiance spectra received by the samples were calculated by multiplication of the original spectrum of the lamp with the transmission spectra of the acrylic and cut-off filters. The resulting spectra for the UV-range are given in Fig. 1. Differences in spectral irradiance between pairs of filters with subsequently shorter cut-off wavelengths were calculated by subtracting wavelength by wavelength each pair of neighboring irradiance spectra. The organisms were kept under continuous illumination for up to 72 h. Samples for HPLC analysis were taken at the start and the end of the experiments, and samples for spectroscopic analysis at the start and end of the experiment (experiment 1) and at 24 h intervals (experiment 2).
Fig 1: Irradiance spectra produced by different combinations of the solar lamp and
cut-off filters.
Spectroscopic analysis
Samples of 1.5 ml cell suspension were centrifuged 10 min at 9000 x g. The pellet was
extracted over night in darkness in 750 µl 100 % methanol at 4 °C.
Absorption spectra were recorded in the range between 220 nm and 750 nm using a single
beam spectrophotometer (DU 70, Beckman, Palo Alto, USA).
HPLC analysis
Cells were harvested by centrifugation at 5000 x g for 10 min at room temperature. Samples were extracted in 5 ml of 20 % (v/v) aqueous methanol (HPLC grade) by incubating at 45 °C for 2.5 h. After centrifugation (5000 x g; GP centrifuge, Beckman, Palo Alto, USA) the supernatant was lyophilized (Lyovac GT 2, Leybold, Köln, Germany) and redissolved in 750 µl 100 % methanol, vortexed and centrifuged at 10 000 x g for 10 min (Sigma2-MK, Sigma Laborzentrifugen GmbH, Osterode, Germany). Thereafter a 700 µl aliquot of the supernatant was evaporated to dryness at 45 °C and the resdidue redissolved in 250 µl ml of water. The samples were filtered through 0.2 µm pore-sized microcentrifuge filters (Mikro-Spin Zentrifugenfilter, Roth, Karlsruhe, Germany).
Further analysis was performed by HPLC [11] using a LiCrospher RP 18 column and guard (5 µm packing; 250 4 mm I.D.) and a mobile phase of 0.02 % acetic acid at a flow rate of 1.0 ml min-1. Detection was done using a phododiode array detector (Waters 990, Waters, USA) in the wavelength range between 280 and 400 nm. Identification of the MAAs was done by comparing the absorption spectra and retention times with several standards kindly provided by Dr. Ulf Karsten, Alfred-Wegener-Institut, Bremerhaven, Germany.
Results
Absorption spectroscopy
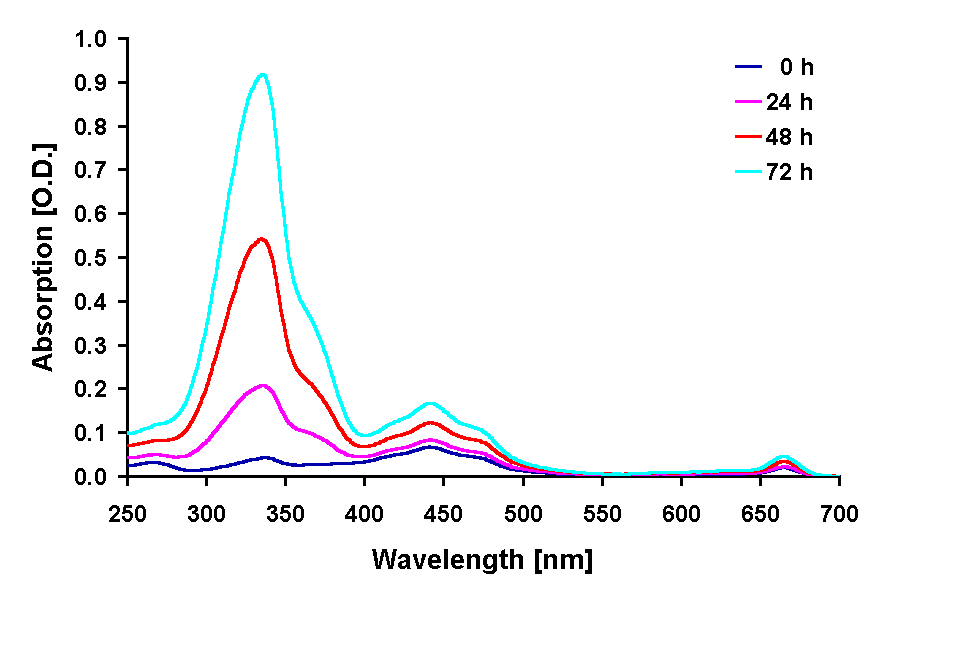
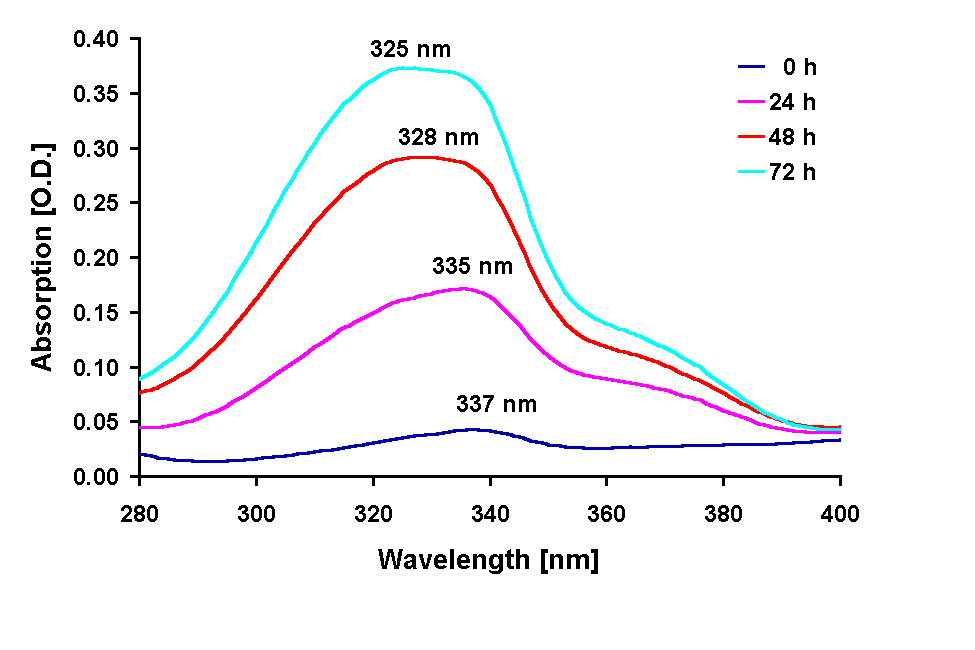
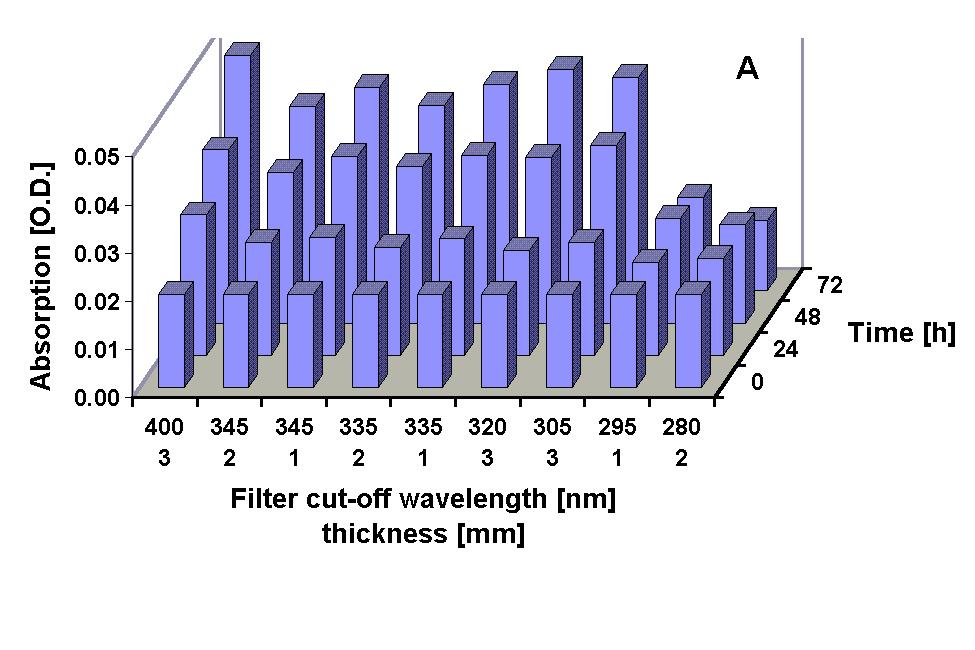
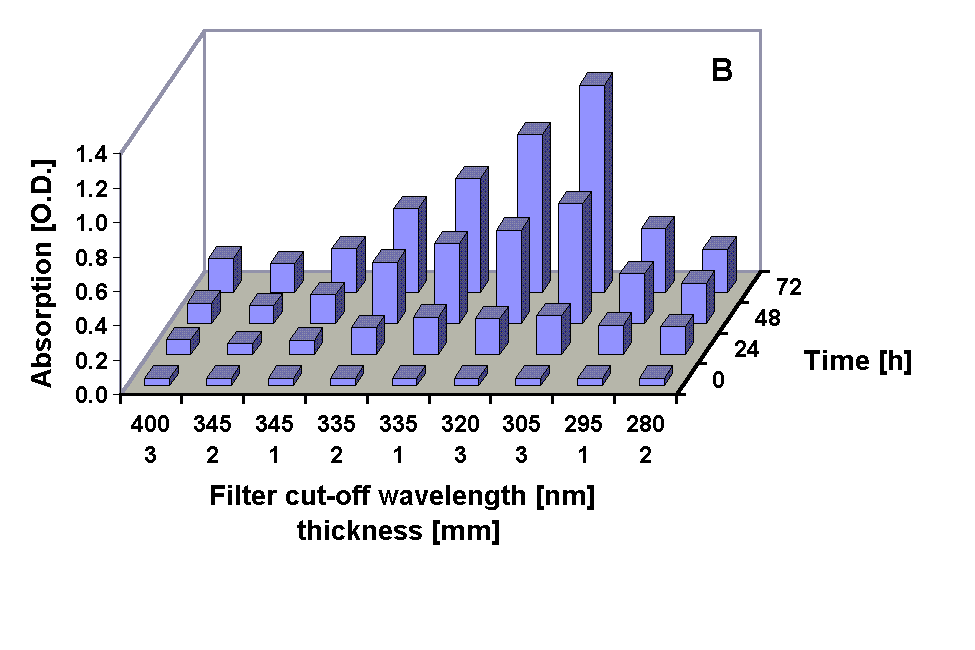
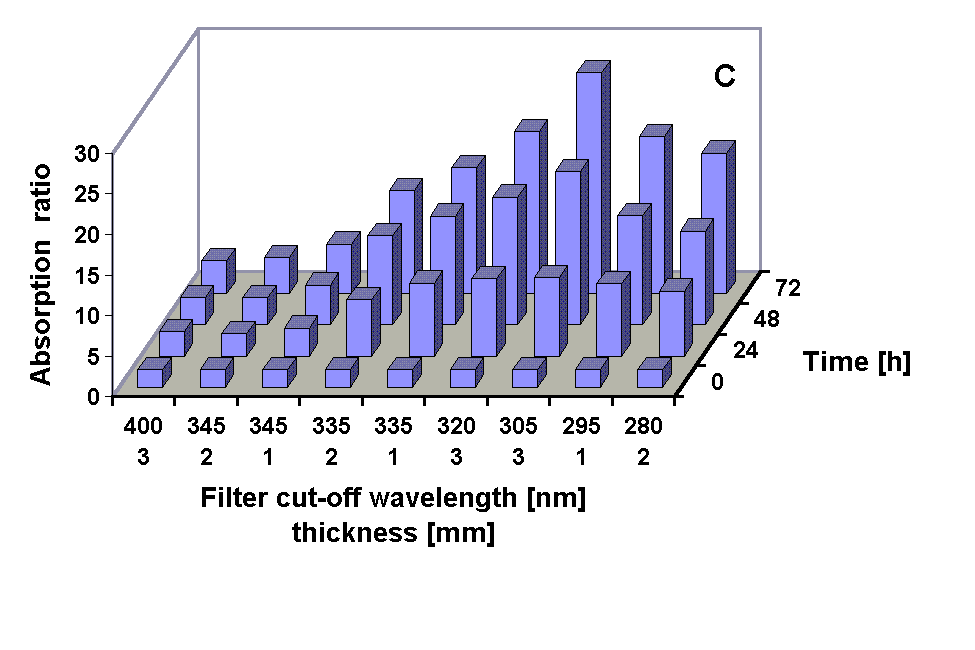
Absorption spectra of the 100 % methanolic extract showed an increase in the absorption at 665 nm (Chl a) in all treatments except under the 295 and 280 nm cut-off filter treatment were the Chl a absorption was decreased after 72 h. A pronounced increase in the absorption in the UV region during the experiments occurred in all treatments (Figs. 2, 3 and 4 B) The UV-absorption peak was at around 336 nm in all samples except those from the 295 nm and 280 nm cut-off filter treatment, where the peak absorption shifted to shorter wavelengths during exposure (Fig. 3). The increase in UV absorption in the extracts was enhanced under shorter cut-off wavelength filters down to 305 nm. Under the 295 and 280 nm cut-off filters the UV peak absorption increased to a lower degree then under the 335 nm to the 305 nm filter. Applying the ratio of the UV peak absorption and the Chl a absorption as a relative measure of the concentration of UV absorbing compounds it can bee seen that the decreased MAA production in the short wavelength treatments (295 nm and 280 nm) is less dramatic (Fig. 4 C).
Fig. 2: Changes in the absorption of methanolic extracts from G. dorsum
exposed to solar lamp under a WG 305 cut-off filter for up to 72 h.
Fig. 3: Changes in the spectral shape of the absorption of methanolic extracts from
G. dorsum exposed to a solar lamp under a WG 295 cut-off filter for different
intervals. The peak absorption wavelength is given above the spectra.
Fig. 4: Chl a absorption (A), UV peak absorption (B) and the ratio of UV peak absortion to Chl a absorption in methanolic extracts from G. dorsum exposed to solar lamp under different cut-off filters for up to 72 h.
HPLC analysis
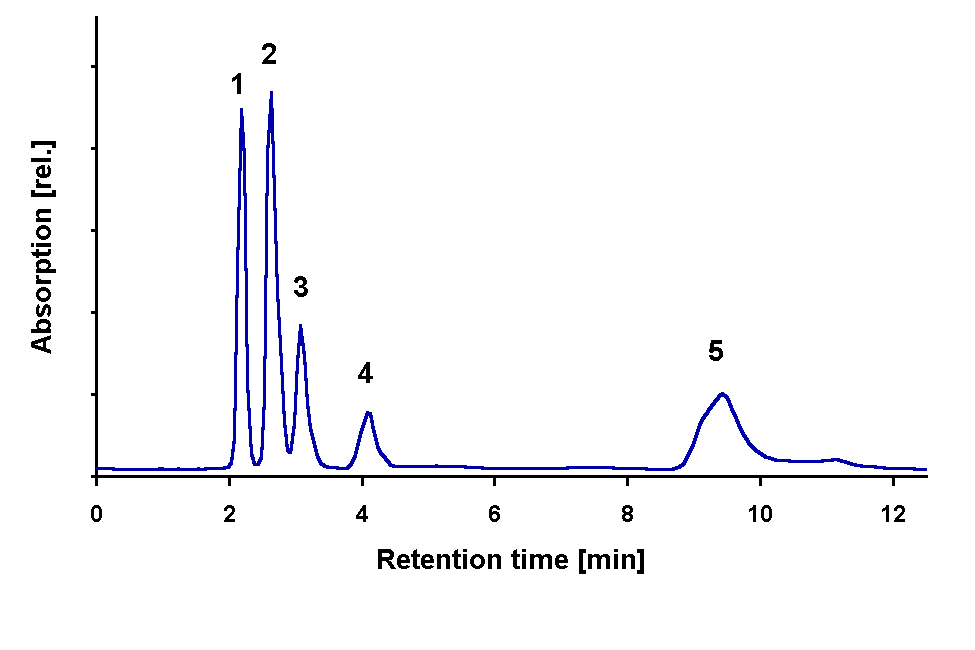
HPLC analysis revealed that the samples contain a complex mixture of MAAs. Three of the
compounds present could be tentatively identified by comparison of retention times and
absorption spectra as shinorine (fraction 1, RT 2.1 min) , porphyra-334 ( fraction 2, RT
2.6 min), and palythine (fraction 4, RT 4.1 min) . The peak at 3.1 min (fraction 3) had an
absorption maximum at the same wavelength as mycosporine-glycine (310 nm) but a different
retention time. The shape of the peak at 9.5 min indicates that it results from an
incompletely separated mixture of at least three compounds (Fig. 5).
Shinorine (fraction 1) was not completely separated from compounds with strong absorption
below 300 nm tailing into the longer wavelength range.
Fig. 5: Chromatographic separation of MAAs from G. dorsum exposed to solar lamp under a WG 305 cut-off filter for 72 h.
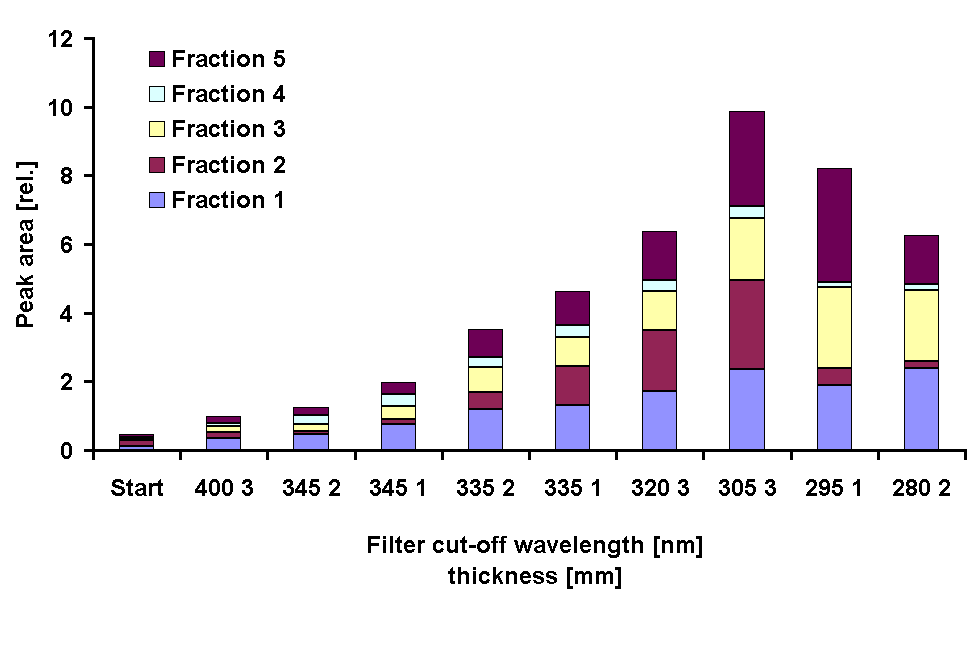
For a relative measure of the contribution of the different fractions to the total MAA amount, the integrated peak area at the maximum absorption wavelength of each peak (334 nm for fraction 1) was used. The total peak area of the treatments showed the same pattern as the extent of the UV absorption peak obtained in spectroscopic analysis: an increase down to a cut-off wavelength of 305 nm and lower values under the 295 nm and 280 nm cut-off filters (Fig. 6).
The pattern of MAA composition among the different treatments was congruent in both experiments. The proportion of palythine increased when long wavelength UV-A was included in the irradiance spectrum (cut-off 345 nm) and decreased when shorter wavelengths were added (cut-off < 345 nm). Porphyra-334 contributed less to the total peak area when UV-A radiation was included but increased when shorter wavelengths were added down to a cut-off wavelength of 305 nm. Under the 295 nm and 280 nm filter the part of fraction 2 (porphyra-334) almost disappeared in comparison to all other cut-off filters.
Fig. 6: Peak areas of the fractions obtained in HPLC chromatograms obtained from G. dorsum before and after exposure to a solar lamp under different cut-off filter for 72 h. The peak areas were normalized to the Chl a concentration of the samples.
Discussion
G. dorsum is an organism adapted to high light environments. The organism exhibits a positive phototaxis even at the highest fluence rates and is hardly prone to photobleaching by high intensity white light [12]. The accumulation of high amounts of MAAs in G. dorsum in response to high irradiance is a feature likely contributing to the adaptation of this organism to conditions of high irradiance as they occur near surface in their natural habitat. Although UV radiation, especially UV-B, in coastal waters is strongly attenuated in the water column [13] due to the phototactic behavior, the organism is likely to be exposed to significant doses of UV-A and UV-B radiation. As a consequence of their absorption properties most MAAs provide the strongest protection in the UV-A range. Cultures of the dinoflagellate Gymnodinium sanguineum containing high amounts of MAAs, in contrast to those with low MAA concentration, are almost insensitive to photoinhibition of photosynthesis by radiation in the wavelength range between 320 nm and 360 nm [14]. The increase in Chl a during the experiments in the present study was not or only slighly affected by the high level of UV-A that was applied. Only under the 295 nm and 280 nm cut-off filters which transmit considerable amounts of UV-B radiation, the Chl a level remained almost constant during the experiment (295 nm cut-off), respectively declined (280 nm cut-off) indicating severe inhibition of growth by short wavelength radiation.
The irradiance spectra produced by the combinations of the solar lamp and the set of filters used in the experiments exhibit broad overlaping regions of differing spectral irradiance (Fig. 1) so that conclusions from the present data on spectral effects of radiation cannot be drawn with great precision. However the large difference in long wavelength UV-A radiation between the 400 nm cut-off filter and the 345 nm cut-off filters produced only a small increase in the ratio of UV peak absorption to Chl a absorption in methanolic extracts and in total peak area of chromatograms.
In marine organisms so far only two aminocyclohexenone MAAs have been identified: mycosporine-glycine and mycosporine-taurine [15]. The shorter retention time of the fraction 3 in G. dorsum indicates that this substance might be the more polar mycosporine-taurine. However to prove this, chemical characterization would be necessary which is beyond the scope of this study.
The change in MAA composition under the short wavelength cut-off filters (295 and 280 nm) leads to a relative increase in the absorption of UV-B radiation. However, considering the decrease of one specific MAA (porphyra-334) it seems likely that there is either a specific inhibition of the synthesis of or this compound or porphyra-334 is selectively metabolized under the short wavelength UV radiation. Another factor possibly causing changes in the MAA composition is the nitrogen nutritional status of the organisms. The UV absorption peak in methanolic extracts of G. dorsum from stationary growth phase or that have been transferred to nitrogen free medium is shifted to shorter wavelengths (unpublished data), indicating that there is a change in the MAA composition. It is known that UV-B radiation leads to reduced uptake of NO3- by planktonic algae [16], so that the observed effect might be an indirect effect, mediated by reduced availability of nitrogen for the biosynthetic pathway of MAA synthesis.
Acknowledgements
This work was financially supported by the European Union (DGXII, Environment
programme, ENV4-CT97-0580) to D.-P. Häder. We would like to thank Almut Gröniger for the
spectroradiometric measurements.
[1]
Häder D.-P. 1988, Ecological consequences of photomovement in microorganisms, J. Photochem. Photobiol. B: Biol. 1, 385-414.[2] Karentz, D., Cleaver, J. E., Mitchell, D. L. 1991, DNA damage in the Antarctic, Nature 350, 28.
[3] Xiong, F., Komenda, J., Kopecky, J., Nedbal, L. 1997, Strategies of ultraviolet-B protection in microscopic algae, Physiol. Plant. 100, 378-388.
[4] Carreto J. I., Carignan M. O., Daleo G., De Marco, S. G. 1990, Occurence of mycosporine-like amino acids in the red tide dinoflagellate Alexandrium excavatum: UV-protective compounds?, J. Plankton Res. 12, 909-921.
[5] Vernet M., Whitehead K. 1996, Release of ultraviolet-absorbing compounds by the red-tide dinoflagellate Lingulodinium polyedra, Mar. Biol. 127, 35-44.
[6] Helbling, E. W., Chalker, B. E., Dunlap, W. C., Holm-Hansen, O., Villafañe, V. E. 1996, Photoacclimation of Antarctic diatoms to solar ultraviolet radiation, J. Exp. Mar. Biol. Ecol. 204, 85-101.
[7] Dunlap, W. C., Rae, G. A., Helbling, E. W., Villafañe, V. E., Holm-Hansen, O. 1995, UV-absorbing compounds in natural assemblages of Antarctic phytoplankton, Antarct. J. U. S. 30, 323-326.
[8] Hannach, G., Sigleo, A. C. 1998, Photoinduction of UV-absorbing compounds in six species of marine phytoplankton, Mar. Ecol. Prog. Ser. 174, 207-222.
[9] Carreto J.,I.,. Lutz, V. A., De Marco, S. G., Carignan, M. O. 1990b, Fluence and wavelength dependence of mycosporine-like amino acid synthesis in the dinoflagellate Alexandrium excavatum, In: Graneli, E., Edler, L., Sundström, B., Anderson, D. M. (eds.), Toxic marine phytoplankton, Elsevier, New York, 275-279.
[10] Guillard, R. R. L., Ryther, J. H. 1962, Studies of marine planktonic diatoms. I. Cyclotella nana Hustedt and Detonula convervacea (Cleve) Gran., Can. J. Microbiol. 8, 229-239.
[11] Nakamura, H., Kobayashi, J. 1982, Separation of mycosporine-like amino acids in marine organisms using reversed-phase high-performance liquid chromatography, J. Chromat. 250, 113-118.
[12] Ekelund, N., Häder, D.-P. 1988, Photomovement and photobleaching in two Gyrodinium species, Plant Cell Physiol. 29(7), 1109-1114.
[13] Piazena, H., Häder, D.-P. 1994, Penetration of UV radiation in coastal lagoons of the southern Baltic Sea and its effect on phytoplankton communities, Photochem. Photobiol. 60, 463-469.
[14] Neale, P. J., Banaszak, A. T., Jarriel, C. R. 1998, Ultraviolet sunscreens in Gymnodinium sanguineum (Dinophyceae): mycosporine-like amino acids protect against the inhibition of photosynthesis, J. Phycol. 34, 928-938.
[15] Bandaranayake, W. M. 1998, Mycosporines: are they nature's sunscreens?, Natural Product Reports, 159-172.
[16] Döhler, G. 1992, Impact of UV-B radiation on uptake of 15N-ammonia and 15N-nitrate by phytoplankton of the Wadden Sea, Marine Biol. 112, 485-498.
[17] Riegger, L., Robinson, D. 1997, Photoinduction of UV-absorbing compounds in Antarctic diatoms and Phaeocystis antarctica, Mar. Ecol. Prog. Ser. 160, 13-25.