| Papers and Posters | Site Home Page |
PREVITAMIN D CONFORMATIONS AND ESTIMATION ON THEIR ROLE IN THE WAVELENGTH DEPENDENCE OF PREVITAMIN D PHOTOSYNTHESIS IN VITRO
Olga Dmitrenko1, John H. Frederick2 and Wolfgang Reischl3
1)
Institute of Surface Chemistry, National Academy of Sciences of Ukraine, 252022 Kiev, Ukraine (current address: Department of Chemistry and Biochemistry, Brown Laboratory,University of Delaware, DE 19716, odmitr@udel.edu)
2)
Department of Chemistry/216, University of Nevada, Reno, NV 89557 USA,jhf@chem.unr.edu
3)
Institute of Organic Chemistry, University of Vienna, A-1090 Vienna, AustriaA8401DAM@helios.edvz.univie.ac.at
Introduction
The UV part of sunlight which penetrates the earth´s atmosphere can cause beneficial and detrimental effects on living organisms [1]. The vitally important vitamin D synthesis is induced by natural UV-irradiation in the epidermis [2]. This reaction sequence involves the photochemical ring-opening of the steroidal precursor 7-dehydrocholesterol (provitamin D) to previtamin D and subsequent thermal rearrangement ([1,7]-hydrogen migration) to the prohormone vitamin D [3].
In solutions, photosynthesis of previtamin D (Figure 1) is a complex branched network of reversible and irreversible isometrization reactions, with previtamin D occupying central position. Besides of the cis-trans isomerization into tachysterol and naturally occurring ring-closure into lumisterol or provitamin D, irreversible photochemical formation of the over-irradiation products (toxisterols, Tox) takes place [4,5].
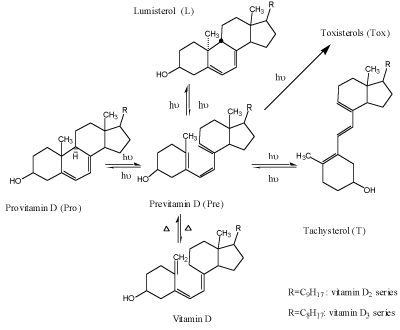
Figure 1. Scheme of provitamin D photoisomerization
Provitamin D and its main photoisomers absorb in the same UV-region (Figure 2) and being excited they all interconvert by photoisomerizations (Figure1). In addition previtamin D and tachysterol may undergo irreversible conversions into toxisterols with different efficiencies. This results in complex reaction mixtures which composition strongly depends on the wavelength of irradiation applied [6-8 and references therein] and the reaction medium [4].
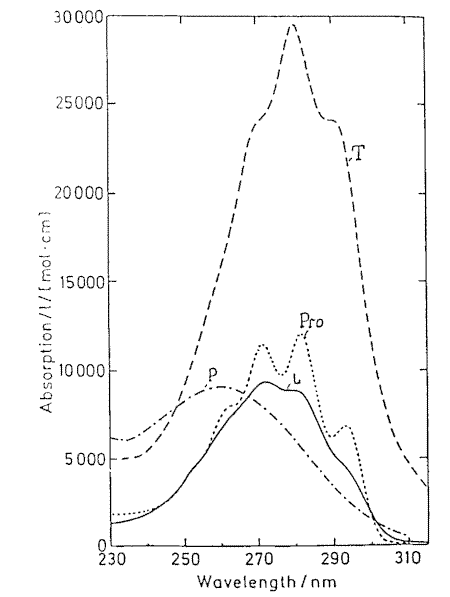
Figure 2. Absorption spectra of previtamin D photoisomers
Mainly, the wavelength dependence in previtamin D photosynthesis is caused by different absorbance of the photoisomers involved in the reaction network [6-7].
There is another, more complicated wavelength effect in the previtamin D photochemistry. It arises from distinct changes observed in previtamin D photoreactions: a sudden increase in the efficiency of ring-closure reactions and the decrease of Z/E isomerization efficiency between 302 and 305 nm [7c-e] (see Table1).
Table 1. Quantum yields of previtamin D photoconversions
nm |
Pre-->Lumisterol |
Pre-->Pro |
Pre-->T |
ref. |
253.7 |
0.04 |
0.014 |
0.41 |
[9] |
302 |
0.09 |
0.02 |
0.27 |
[9] |
0.09 |
0.04 |
0.27 |
[6d] |
|
305.0 |
0.18 |
0.08 |
0.29 |
[6d] |
Several models have been proposed to explain this dramatic changes in quantum yields :
1) ground state conformational control (selective excitation of different conformers possessing different absorption spectra and photoreactivity) [10]: Due to the high flexibility of the previtamin D´s chromophore [11, 12] and its non-planar geometry [11-14] the direct measurement of individual absorption spectra of conformers is unachievable and some experimental strategies like low temperature matrix techniques and computational separation of the individual spectra of the averaged solution spectra have been pursued [7c]. No marked difference between spectra of cZc conformer (the primary one formed after ring-opening from provitamin D) and the extended tZc conformers has been demonstrated experimentally [15]. Recent gas-phase semi-empirical calculations [13,14] predict the difference in 0-0 transitions of cZc and tZc conformers, thereby strongly supporting the idea of the ground state conformational control as possible origin of the wavelength effect. cZc conformers can be selectively excited at the very red edge of previtamin D absorption band (which is actually a sum of individual conformer contributions), whereas tZc ones remain unactive.
2) twin-state model: This explanation involves the crossing of the 1B2 and 2A1 excited states [16,17]. It has been postulated that after being excited to the strongly allowed 1B2 state previtamin D may follow two routes of relaxation on its excited state potential energy surface - one is the direct path to the ground state surface of the E-isomer, the other is the decaying to the 2A1 state, which leads to ring closure. With wavelengths longer than 305 nm, the 2A state is directly excited and the channel to the E-isomer is no longer available. This postulate has been supported experimentally by fluorescence studies that involved numerous assumptions and mathematical treatments [16].
3) hot reactions in the excited state: One of two competing isomerizations occur over a barrier in the exited state., The larger the energy of the excited molecule the more probable it will traverse this barrier making this pathway more efficient [7c,f]. This mechanism has been proposed to explain not only the sudden change of quantum yields (see above) but also their variation in the long-wavelength region [7c]. Nevertheless, the role of conformational control is not denied by authors [7c]. This model violates the NEER principle (conformational control) [4] which does not seem unlikely in view of the recent postulate of a hula-twist isomerization mechanism [18].
In this study we will examine the first mechanistic model in order to get an idea on how the conformational flexibility of previtamin D may contribute to the change of quantum yields observed. In particular, we will apply theoretical approach from [19] to the photoreaction model with conformationally flexible intermediate photoisomer (Figure 3) and modify it for the case of previtamin D photoisomerizations to get the equation for the ratio of quantum yields of cis-trans and ring-closure isomerizations. Characteristics on previtamin D confirmers obtained by density functional (B3LYP/6-31G(d)) and QCFF semi-empiric computations will be used for quantitative estimation of the ratio which then will be compared with the experiment [16b].
Theoretical modeling of the photoreaction quantum yields ratio
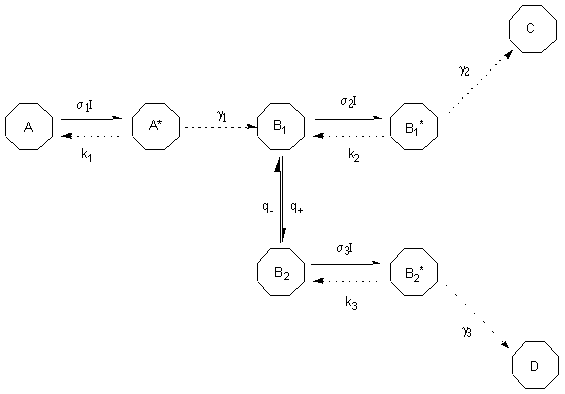
Figure 3. Model of two-product photolysis reaction involving 2 conformations of the intermediate isomer. Here s i are the absorption cross-sections (cm2) and I is the photon flux (photon cm-2 s-1). ki ( s-1) and g i ( s-1) characterize the non-reactive and reactive transitions, respectively.
For case of low-intensity irradiation (lamp irradiation, for instance), the ratio of quantum yields of photoproducts C and D formation was found as following [19c]:
fC/fD=q-
s 2h 2/ q+s 3h 3 (1)Here h i = g i /( ki + g i ) is characteristic of the excited state. Ratio q-/ q+ determines the conformational equilibrium at the ground state and in further can be substituted by the ratio of conformational populations.
Generalization of the above case for the multiconformational ground state equilibrium of the intermediate photoisomer (where one set of confirmers (i) are precursors of product C whereas another set (j) leads to D) results in:
fC/fD=å pi
s ih i/ å pjs jh j (2)As it was found by different modeling methods [11-14], previtamin D has at least 8 stable ground state conformations that differ in configuration around single bonds ( C5-C6 and C7-C8), sign of the corresponding dihedral angles and A-ring conformation (axial and equatorial orientation of 3b -OH). According to generally accepted assumption based on NEER principle and illustrated in Figure 5, one may re-write formula (2) for the specific case of previtamin D photoconversion :
fcis->trans/fring-closure=å pt
s th t/ å pcs ch c (3)Here indexes ‘t’ and ‘c’ correspond to tZc and cZc conformers, respectively. fcis->trans is quantum yield of Pre -> T Z/E isomerization, fring-closure we define as sum of Pre->Pro and Pre->L ring-closure quantum yields.
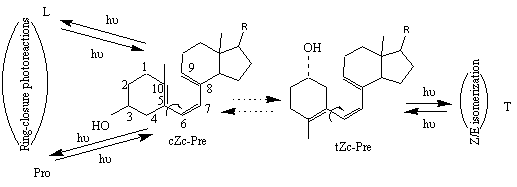
Figure 5. Simplified two-conformation scheme of previtamin D reversible photoconversions. Here and below, Z denotes cis geometry in relation to the C6-C7 double bond. Letters c and t refer to the s-cis and s-trans conformations of the C5-C6 and C7-C8 single bonds.
If to assume for simplicity that h t=h trans, h c=h cis and h trans/ h cis=Const, one may obtain working equation (4) that will be used below when comparing the simulating results with the experimental dependence.
fcis->trans/fring-closure=Const * å pt
s t/ å pcs c (4)
Results of a computational study by density functional theory (B3LYP/6-31G(d)) of prevititamin D ground state conformational distribution.
For the calculation of conformational populations we use here the results of b3lyp/6-31G(d) [20] gas-phase optimizations (Table 2) in conjunction with the fact that in solvent 1,2-dimethylcyclohexene (A-ring model) prefers equatorial 4-OH for 0.7 kcal/mol with respect to axial [21] (this value was added to the relative axial conformer energies).
conformer |
E, a.u. |
rel.E, kcal/mol |
rel.E solv. kcal/mol |
conformer abundance in solvent, % |
Dipole, Debye |
3b -H equatorial |
|||||
(–)cZ(–)c |
-855.373905 |
1.68 |
3.60 |
1.6836 |
|
(+)cZ(+)c |
-855.375078 |
0.95 |
12.34 |
1.3281 |
|
(–)tZ(+)c |
-855.375920 |
0.42 |
29.88 |
1.1419 |
|
(+)tZ(–)c |
-855.375741 |
0.53 |
24.76 |
1.3126 |
|
3b -H axial |
|||||
(–)cZ(–)c |
-855.374482 |
1.32 |
2.02 |
2.04 |
2.2348 |
(+)cZ(+)c |
-855.374525 |
1.29 |
1.99 |
2.14 |
1.7568 |
(–)tZ(+)c |
-855.375603 |
0.62 |
1.32 |
6.64 |
1.8163 |
(+)tZ(–)c |
-855.376584 |
0 |
0.70 |
18.60 |
1.4446 |
Simulations of UV spectra and fcis->trans/fring-closure wavelength dependence
Conformer absorption cross-section is proportional to the oscillator strength and depends on wavelength of irradiation as its absorption spectrum does. One may write:
s i=Const* fi*Ri(l )
(5)where Ri(l ) characterizes absorption bandshape of i-conformer.
Absorption of previtamin D is a sum of individual conformer contributions, and the total absorption spectrum S(l ) can be modeled according to equation (6):
S(l )=Const*S s ipi
(6)In order to calculate previtamin D absorption bandshape we have used an assumption that each conformer has gaussian bandshape with maximum at Franck-Condon transition wavelengths and halfwith equal ½(l 0-0 - l FC), where l 0-0 and l FC are wavelengths of origin 0-0 and vertical Franck-Condon transitions, respectively. These characteristics of first strongly allowed previtamin D excited state have been calculated earlier in [13]. In Figure 6, such a simulation of previtamin D spectrum and contributions of cZc and tZc conformers are presented.
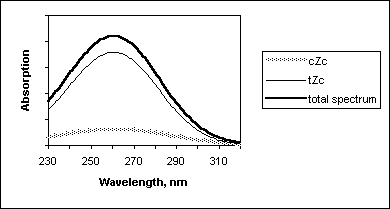
Figure 6. Calculated absorption spectrum of previtamin D and contributions of its cZc and tZc conformers.
Nevertheless, this so simplified approach resulted in good agreement with the experimental absorption spectrum of previtamin D (Figure 7).
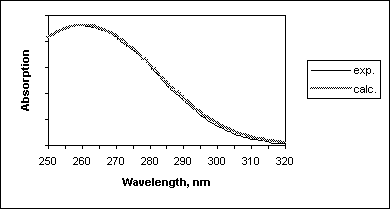
Figure 7. Comparison of the experimental and calculated spectra of previtamin D.
Using data on these spectral calculations and formula (4) the ratio of cis-trans quantum yield to ring-closure quantum yield has been calculated and compared with the experimental ratio
(qPre->T/(qPre->Pro +qPre->L)). Figure 8 illustrates the results of this comparison.
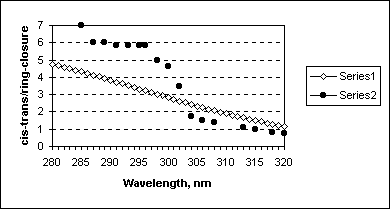
Figure 8. Comparison of calculated fcis->trans/fring-closure (Series1) with experimental qPre->T/(qPre->Pro +qPre->L) (Series 2, data are taken from [16b]).
Conclusions
In this study we have found that cZc conformers has markedly smaller contribution in the absorption of previtamin D (excluding red egde of the absorption band).
In the range 303-320 nm we have perfect agreement of the theoretical and experimental ratios of the quantum yields (up to the constant in equation 4), whereas shorter wavelength irradiation results in higher ratio and different dependence. This indicates that at wavelengths larger 303 nm the reaction is following classical NEER way (or conformationally controlled), while at higher energies of excitations another mechanisms may be responsible for the previtamin D photoconversions channels.
Acknowledgments
We are grateful to Dr.W.Fuss, Dr.J.T.Vivian and Dr.I.P.Terenetskaya for valuable and stimulating discussions. Support from NSF(CHE-9419102 to J.H.F.) and Österreichische Nationalbank (Jubiläumsfondsprojekt Nr.:7395/1 to W.R.) is gratefully acknowledged.
REFERENCES
1. (a) Biological Effects of Light, M.F. Holick and A.M. Kligman (eds.), 1992, Walter de Gruyter, Berlin - New York (b) S. Beissert and R.D. Granstein, (1996) UV-Induced Cutaneous Photobiology, Crit. Rev. Biochem. Mol. Biol. 31, 381-404;
2. (a) M.F. Holick, Photobiology of Vitamin D, in: Vitamin D, D. Feldman, F.H. Glorieux, J.W. Pike (eds.), 1997, Academic, San Diego, California, 33-39; (b) J.A. MacLaughlin, R.R. Anderson, M.F.Holick, (1982) Spectral Character of Sunlight Modulates Photosynthesis of Previtamin D and its Photoisomers in Human Skin, Science 216, 1001-1003; (c) M.F. Holick, J.A. MacLaughlin and S.H. Doppelt, (1981) Regulation of Cutaneous Previtamin D3 Photosynthesis in Man: Skin Pigment is not an Essential Regulator, Science 211, 590-593; (d) R.R. Anderson, Tissue Optics and Photoimmunology, in: Photoimmunology, J.A. Parrish, M.L. Kripke, W.L. Morrison (eds.), 1983, New York: Plenum Press, 61-76;
3. (a)E. Havinga, (1973) Vitamin D, Example and Challenge, Experientia 29, 1181-1316; (b) R. Bouillon, W.H. Okamura, A.W. Norman, (1995) Structure-Function Relationships in the Vitamin D Endocrine System, Endocrine Rev. 16, 200-257;
4. (a) H.J.C. Jacobs and E. Havinga, (1979) Photochemistry of Vitamin D and its Isomers and of Simple Trienes, Adv. Photochem.11, 305-373; (b) H.J.C. Jacobs, F. Boomsma, E. Havinga, A. Van der Gen, (1977) Studies on Vitamin D and Related Compounds. Part XXVII. The Photochemistry of Previtamin D and Tachysterol, Rec. Trav. Chim. Pays-Bas 96, 113-117; (c) F. Boomsma, H.J.C. Jacobs, E. Havinga, A. Van der Gen, (1977) The "Overradiation Products" of Previtamin D and Tachysterol: Toxisterols, Rec. Trav. Chim. Pays-Bas 96, 104-112; (d) J.W.J. Gielen, The Photochemistry of Vitamin D and Related Conjugated Trienes, Ph. D. Thesis, Leiden, 1981; (e) A.G.M. Barrett, D.H.R. Barton, (1977) Photochemical Transformations. Part 34. Structures of Toxisterols, J. Chem. Soc. Perkin I 631-642;
5. P.A. Maessen, The Formation of Toxisterols from Previtamin D. Mechanistic Studies, Ph. D. Thesis, Leiden, 1983;
6. (a) K. Pfoertner, J.P. Weber, (1972) Photochemistry in the vitamin D series. I. Kinetics and Quantum Yields of Ergosterol Irradiation at 253.7 nm, Helv. Chim. Acta 55, 921-937; (b) K. Pfoertner, (1972) Photochemistry in the vitamin D series. II. Influence of the wavelengths on the photoisomerization of precalciferols, Helv. Chim. Acta 55, 937-947; (c) R. Mermet-Bouvier, E. Abilon, (1973) Ergosterol Photoisomerization Reaction Scheme, J. Pharm. Sci. 62, 891-894;
7. (a) M. Braun, W. Fuss and K.L. Kompa, Improved Photosynthesis of Previtamin D by Wavelengths of 280-300 nm, (1991) J. Photochem. Photobiol. A: Chem. 61, 15-26; (b) N. Gottfried, W. Kaiser, M. Braun, W. Fuss, K.L. Kompa, (1984) Ultrafast Electrocyclic Ring Opening in Previtamin D Photochemistry, Chem. Phys. Lett. 110, 335-339; (c) W. Fuss, S. Lochbrunner, (1997) The Wavelength Dependence of the Photochemistry of Previtamin D, J. Photochem. Photobiol., A.: 105, 159-164; (d) W.G. Dauben, P.E. Share, R.R. Ollmann, (1988) Triene Photophysics and Photo-chemistry: Previtamin D3, J. Am. Chem. Soc.110, 2548-2554; (e) P.E. Share, Polyene Photophysics and Photochemistry: Previtamin D3, Ph. D. Thesis, Berkeley, 1985; (f) W. Fuss, S. Lochbrunner, A.M. Muller, T. Schikarski, W.E. Schmid, S.A. Trushin, (1998) Pathway Approach to Ultrafast Photochemistry: Potential Surfaces, Conical Intersections and Isomerizations of Small Polyenes, Chem. Phys. 232, 161-174;
8. (a) I.P. Terenetskaya, S.I. Gundorov, V.I. Kravchenko, E.B. Berik, (1988) Nanosecond Laser Photolysis of Provitamin D, Kvantovaya Elektron. 18, 1323; (b) N.A. Bogoslovsky, E.B. Berik, S.I. Gundorov, I.P. Terenetskaya, (1989) Characteristics of Laser Photolysis of Provitamin D, High Energy Chem. 23, 218; c) I.P. Terenetskaya, S.I. Gundorov, E.B. Berik, (1991) Characteristics of Photolysis of Provitamin D by Long-Wavelength Radiation, Kvantovaya Elektron. 21, 472; (d) Y.A. Repeev, I.P. Terenetskaya, (1991) Laser Photosynthesis od Previtamin D: New Effects of High-Intensity Picosecond Irradiation, Kvantovaya Elektron .23: 765-768;
9. (a) W.G. Dauben and R.B. Phillips, (1982) Wavelength Controlled Production of Previtamin D, J. Am. Chem. Soc.104, 355-356; (b) V. Malatesta, C. W. Willis and P.A. Hackett, (1981) Laser Photochemical Production of Vitamin D, J. Am. Chem. Soc. 103, 6781-6783;
10. (a) H.J.C. Jacobs, J.W.H. Gielen, E. Havinga, (1981) Effects of Wavelength and Conformation on the Photochemistry of Vitamin D and Related Conjugated Trienes, Tetrahedron Lett. 22 , 4013-4016; (b) A.M. Brouwer, J. Cornelisse, H.J.C. Jacobs, (1987) Wavelength Effect on the Photochemical Reaction of (Z)-2,5-Dimethyl-1,3,5-hexatriene, Tetrahedron 43, 435-438; (c) H.J.C. Jacobs, (1995) Photochemistry of Conjugated Trienes: Vitamin D Revisited, Pure & Appl. Chem. 67, 63-70;
11. (a) W.G. Dauben and D.J.H. Funhoff, (1988) Theorertical Evaluation of the Conformations of Previtamin D3, J. Org. Chem. 53, 5070-5075; (b) O. Dmitrenko, and W. Reischl, (1996) Molecular Mechanics-Based Conformational Analysis of Previtamin D and its A-Ring Analogues, Monatshefte f. Chem.127, 445-453;
12. O. Dmitrenko, W. Reischl, (1998) Modeling of Previtamin D Conformational Equilibrium: Effect of Intramolecular Electrostatic Interactions, J. Mol Struc. (Theochem) 431, 229-236;
13. O. Dmitrenko, W. Reischl, J.T. Vivian, J.H. Frederick, Ground and Excited States Properties of Previtamin D Conformers, Refered publication #C5 at Internet site: www.photobiology.com/ v1/contrib.htm, or www.photobiology.com/v1/dmitrenko/ dmitrenko.htm;
14. O. Dmitrenko, W. Reischl, J.T. Vivian, J.H. Frederick, Theoretical Studies of the First Strongly Allowed Singlet States of 3-desoxy Analogs of Previtamin D, Vitamin D and their E-Isomers, J. Mol. Struc. (Theochem), in press;
15. A. Muller, Tieftemperatur-Photochemie von 7-Dehydro-cholesterol und Previtamin D, Diplomarbeit, Technical University of Munich, 1997;
16. (a) W. G. Dauben, P. E. Share, R. R. Ollmann Jr, (1988) Triene Photophysics and Photochemistry: Previtamin D3, J. Am. Chem. Soc. 110 , 2548-2554; (b) W.G. Dauben, B. Disanayaka, D.J.H. Funhoff, B.E. Kohler, D.E. Schilke, B. Zhou, (1991) Polyene 2Ag and 1Bu States and the Photochemistry of Previtamin D, J. Am. Chem. Soc. 113, 8367-8374;
17. S. Zilberg, Y. Haas, (1999) The Singlet-State Photophysics and Photochemistry of Polyenes: Application of the Twin-State Model and of the Phase-Change Theorem, J.Phys.Chem.A. 103,2364-2374;
18. (a) R.S.H. Liu, D.T. Browne, (1986) A Bioorganic View of the Chemistry of Vision: H.T.-n and B.P-n,m Mechanism for Reactions of Confined, Anchored Polyenes, Acc. Chem. Res.19 , 42-48; (b) R.S.H. Liu, A.E. Asato, (1985) Photochemistry of Polyenes 22. The Primary Process of Vision and the Structure of Bathorhodopsin - A Model Study, Proc. Natl. Acad. Sci. USA 82, 259-263; (c) K. Schulten, P. Tavan, (1978) A Mechanism for the Light-Driven Proton Pump of Halobacterium halobium, Nature 272, 85-86; (d) R.S.H. Liu, D. Mead, A.E. Asato, (1985) Application of the H.T.-n Mechanism of Photoismerization to the Photocycle of Bacteriorhodopsin: A Model Study, J. Am. Chem. Soc. 107, 6609-6614; (e) R.H.S. Liu (1998) Looking Back to My Research Effort (1962-1998) www.bgsu.edu/departments/photochem/; Spectrum 11(4), 7;
19. (a) A.A. Serikov, I.P. Terenetskaya, (1994) The Influence of Radiation Intensity on the Pathways of Phototransformations of Conformationally Mobile Molecules, High Energy Chem. (USSR) 28, 257; (b) I.P. Terenetskaya, O.G. Dmitrenko, (1993) Theoretical Basis for the Possibility of the Conformational Control of the Products of Provitamin D Photochemical Conversion, Teoret. i Exper. Khim. 29, 326-332; (c) O.G. Dmitrenko, A.A. Serikov, I.P. Terenetskaya, (1996) Model Analysis of Branching Photoreactions with Conformationally Flexible Intermediate, J. Photochem. Photobiol., A: Chem. 96, 7-12;
20. Theoretical calculations were carried out using the Gaussian94 and Gaussian98 program system: Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Gill, P.M.W.; Johnson, B.G.; Robb, M.A.; Cheeseman, J.R.; Keith, T.; Petersson, G.A.; Montgomery, J.A.; Raghavachari, K.; Al-Laham, M.A.; Zakrzewski, V.G.; Ortiz, J.V.; Foresman, J.B.; Cioslowki, J.; Stefanov, B.B.; Nanayakkara, A.; Challacombe, M.; Peng, C.Y.; Ayala, P.Y.; Chen, W.; Wong, M.W.; Andres, J.L.; Replogle, E.S.; Gomperts, R.; Martin, R.L.; Fox, D.J.; Binkley, J.S.; Defrees, D.J.; Baker, J.; Stewart, J.J.P.; Head-Gordon, M.; González, C.; Pople, J.A. GAUSSIAN 94, Gaussian, Inc., Pittsburgh, PA, 1995.
21. J.B. Lambert, D.E. Marko, (1985) Factors Influencing Conformational Preferences in Cyclohexenes, J. Am. Chem. Soc. 107, 7978-7982;