| Papers and Posters | Site Home Page |
Effect of Light Treatment Conditions on Immunological Mechanisms of Anti-Tumor Resistance.
Vladimir V. Ryl'kov
State Optical Institute, St Petersburg 199034 (Russia)
Fax: (812)2181093 E-mail: ryl@ilph.spb.su
Robert P. Ogurtsov, Valentina P. Puzirjova, Peter G. Nazarov
Institute of Experimental Medicine, St Petersburg 197376 (Russia)
Alexander N. Stukov
Research Institute of Oncology, St Petersburg 189646 (Russia)
Keywords. Adaptation to light, photochemotherapy, photosensitizer, photostimulation of immunity.
The summary
The aim of study was to compare the effects of white light (390-760 nm) and red light (620-760 nm) in photodynamic experiments.
Experiments were performed on mice and cell cultures in vitro. Three photosensitizers in a wide range of doses were used: chlorines e6 and P6, and Photosan-3.
Mice were inoculated with Ehrlich tumor cells, given a sensitizer and then kept in darkness or under the conventional room light. A 3-fold decrease in tumor growth rate was detected in mice, sensitized by dyes and kept on light, as compared with mice protected from light. Influence of red and white light on natural killer cell activity and production of cytokines (tumor necrosis factor [TNF] and interleukin-1 [IL-1]) was investigated in vitro using splenocytes of normal mice and K-562 cells. Mixtures of various compositions were studied: a) splenocytes plus K-562 cells separately dyed with phosensitizers; b) cells dyed plus cells not dyed, and c) cells irradiated plus cells non-irradiated.
The most interesting results were obtained with dyed and light irradiated splenocytes, which were cultured with dyed but non-irradiated K-562 target cells. Survival of K-562 targets showed inverse correlation with photostimulated production of TNF. Photostimulation of natural killer activity and/or TNF production was observed with either of dyes used and was similar with either red or white lightening. The maximum of TNF production and the highest killing of K-562 target cells were seen at doses of light near 1 J/cm2, while the maximum of IL-1 production was associated with light doses, that did not stimulate natural killer activity. At higher doses of light (10-30 J/cm2), the highest enhancement of natural killer activity was achieved by mixing of irradiated dyed targets with non-dyed splenocytes.
We consider two novel aspects of photodynamic therapy of tumors (PDT): (1) phototherapeutic effect of adaptation to natural light of the biological tissues and the organism to natural light; (2) photosensitized stimulation of immune reactions under PDT treatment (new mechanism of photochemotherapy). The results we have obtained are consistent with the inherent photochemistry of living organisms and are based on one common suggestion: all living organisms and their tissues are non-linear photobiochemical systems with feedbacks. The photochemical basis of the evolutionary adaptation to natural light is discussed. We show that the combination of photosensitizer with natural light more selective influence on the organism than irradiation with monochromatic light.
1. Introduction
Previously [1, 2] we have presented experimental data, indicative of involvement of mechanism(s) of photochemical stimulation of immune reactions, in particular under the photodynamic therapy (PDT) procedure of tumor-bearing animals. When mice were inoculated with Ehrlich tumor cells into both hind paws of which only one was treated by light, the second paw (protected from light) changed its size synchronously with irradiated one. In blood of the animals, subjected to the PDT procedure, an increased levels of tumor necrosis factor (TNF-a ) was registered, suggesting that the observed suppression of tumor growth is a result of photostimulated biochemical changes in blood of the animals rather than a consequence of blow-up of tumor blood vessels. In other series of experiments, we treated with light the cultures of bone marrow cells from normal persons and leukemic patients. It was shown, that the more the spectrum of used light differed from the spectrum of natural light, the stronger was the photostimulation of both normal and tumor cell proliferation. Natural light had not practically stimulated proliferative activity of either type of cells. It is noteworthy, that this result was obtained with the cells of bone marrow, which are naturally protected from light in organism. The property of cells not to respond to natural light appears to be a result of their "learning" during evolutionary adaptation to light. This statement can be confirmed by experimental data published by other investigators (see for example [3]). However, we do not know, that others to consider similar data as a result of evolutionary adaptation to natural light.
It had been shown, that standard PDT procedure induces at least two competing processes: the stimulation of tumor growth resulted from the absorption of light by endogenous chromophores when monochromatic sources of light (lasers) are used, and the suppression of tumor growth due to photosensitizer-facilitated response to light. By use of sources of natural light, to which the organisms are adapted, the latter process dominates. The conclusion was made that the combination of photosensitizer with natural light has more selective effect on organism than the combination of photosensitizer with monochromatic light.
2. Materials and methods
Chlorine P6 was a kind gift of Prof. A.F.Mironov (Institute of Fine Chemical Technology, Moscow). Chlorine e6 was obtained from Prof. G.P.Gurinovich (Institute of Physics, Belarus). Prof. H.Mueller von der Haegen (Seehof Laboratorium, Germany) kindly provided us with Photosan-3. Inbred CBA mice weighting from 20 to 23 g were used.. Ehrlich carcinoma cells were injected in the sole pillow of the right and left hind paws. Animals were used on the 4th or 5th day after injection of tumor cells. Dyes (solutions in 0.9% NaCl) were injected intraperitoneally at a doses of 3 - 10 mg per kg of the body weight.
Irradiation of the cell cultures by light was carried out with the xenon arc lamp (120 W) in the range of 390 - 760 nm (white light) and 620 - 760 nm (red light). With the help of mirror optics, the light of the xenon arc lamp was input into the quartz light guide through a set of glass light filters. The light intensity at the target object was controlled by the distance from the exit of light guide. We suppose that the "white" light emitted by the xenon arc lamp with the light filters is similar to natural light.
In experiments on animals, each mouse was labelled individually which enabled us to observe the dynamics of the tumor growth in each mouse separately and to get rid of the error due to possible fluctuations in quantity of the injected tumor cells. Having 10 animals per group, it reduced the error of the relative tumor dimensions from 30 % to 8 %. One and the same person measured the tumor volume (the product of the longitudinal and perpendicular axes times the tumor height) troughout all experiments.
To assess the activity of natural killer cells and the production of tumor necrosis factor (TNF), the mice were subjected to spleen extraction. Then the suspension of splenocytes in medium 199 was prepared using Porter glass homogenizer in medium 199. Splenocytes were washed three times with medium 199 and resuspended to the concentration of 107/ml in medium RPMI 1640 with 10% of fetal calf serum. Splenocytes were incubated with prelabelled with 3H-uridine prelabelled K-562 cells at 20:1 ratio for 24 h at 37 C0. Then the cell mixtures were sedimented by centrifugation for 10 min at 400g. Radioactivity of pellets was measured with Beckman scintillation counter, and indices of cytotoxicity were calculated. In supernatants of cultures, TNF-a and IL-1b were determined. The level of TNF was determined by its cytotoxic action on L-929 cells in the presence of actinomycin D. IL-1 was measured by its costimulatory action on mouse thymocyte proliferation in vitro in the presence of phytohemagglutinin.
3. Results and discussion
3.1. Influence of natural light on experimental tumor growth in mice
If the results of [1, 2] are the case, then the effect of PDT can be obtained with natural light, without use of special light sources. This was confirmed in experiments on mice, sensitized with Chlorine e6 (10 mg/kg) and Photosan-3 (3 mg/kg). Three groups of mice (10 animals in each group), two of them injected with dyes and one with saline (control), were kept in darkness after inoculation with Ehrlich tumor cells. As in [1, 2], tumor cells were inoculated into both hind paws of each animal. Similar three groups of mice were kept at usual room lighting. Tumor growth curves of mice, kept in darkness, did not differ, once again showing that the dyes used are true sensitizers, not effecting the tumor process in darkness. Hereinafter these three groups of mice were pooled and considered as one control group. The tumor growth curve in the group of mice without dye, kept on light, did not differ significantly from tumor growth curve of control group (Figure 1). Inhibition of tumor growth was detected only in groups of mice, sensitized with dyes and kept on light. In all groups of mice, tumor growth kinetics (Figure 2) at saturation (in the middle of period of observation) was rather satisfactory approximated (R2=0,952) by the equation V/Vsat =1 - exp. (- kt) (Figure 3), where t is time. Therefore, the value of tumor growth suppression can be described by coefficient k. The k values were as follows: 0,153 day-1, 0,053 day-1 and 0,050 day-1 for groups of mice without dye, with Chlorine e6 and with Photosan-3, respectively. Exact repetition of this experiment gave the same results. Thus, we have received a 3-fold decrease of tumor growth rates. These results are quantitatively close to those obtained previously when special light sources and standard PDT procedure were applied to only one of tumors [1, 2].
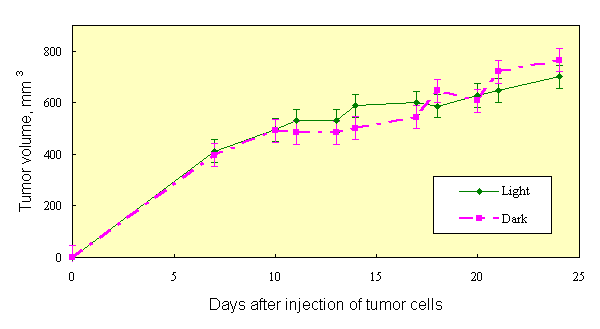
Fig.1. Tumor growth in mice received photosensitizer and kept in dark or at natural light
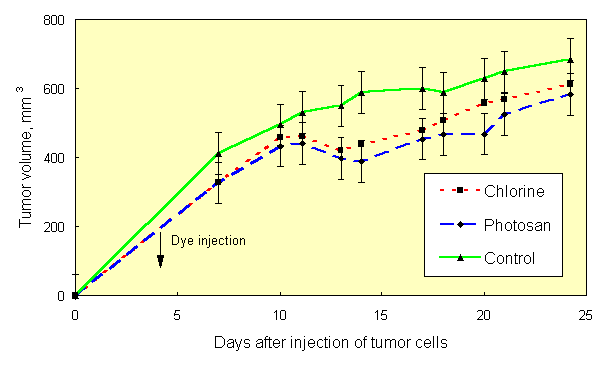
Fig.2. Effect of photosensitizers and light treatment on tumor growth in mice
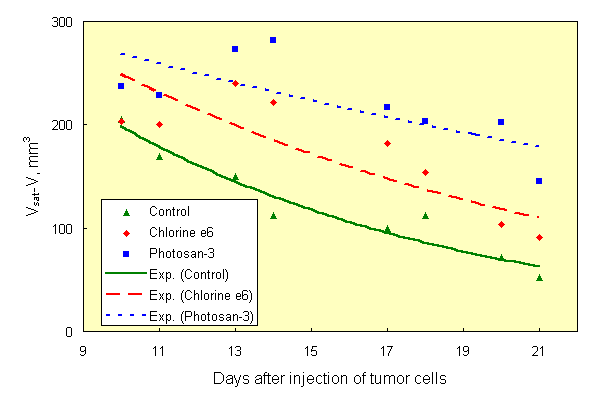
Fig.3. Approximation of tumor growth curves shown on Fig.2 by equation
|
It is necessary to emphasize three points. 1) At present time, optimum conditions of irradiation of blood of the animals for stimulation of immune response are not known. Optimal intensity of light, optimal specific dose of absorbed light, and optimal volume of irradiated blood (total dose of light) are not still estimated. Therefore, quantitatively low inhibitory effects on tumor growth observed in mice do not testify the low efficacy of mechanism of photochemical effect of light on tumor process. 2) In these experiments, the tumors inoculated into hind paws of animals were practically not exposed to light action. A whole body lightening took place instead. Its major effect was mediated most probably via irradiation of blood, which was not interfered by dark skin of the used animals, once again suggesting the evolutionary adaptation of the animals to light. 3) In experiments described in [1, 2], light of low intensity (20-60 mW/cm2) was used, and though the procedure of tumor irradiation was local, almost whole hind leg of animal could be subjected to light action due to light scattering properties of tissues. A large volume of animal's blood could path through it during this time. It, apparently, led to quantitatively close results.
3.2. Influence of light on mixed cultures of splenocytes and K-562 cells.
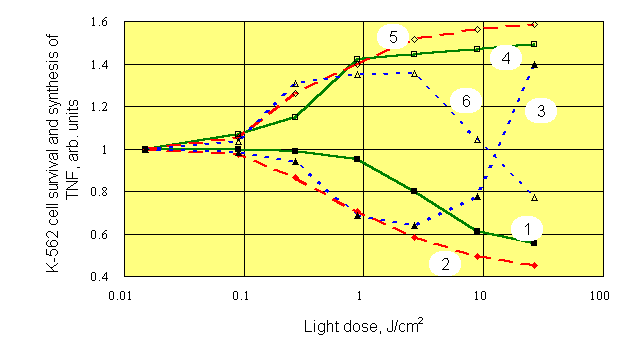
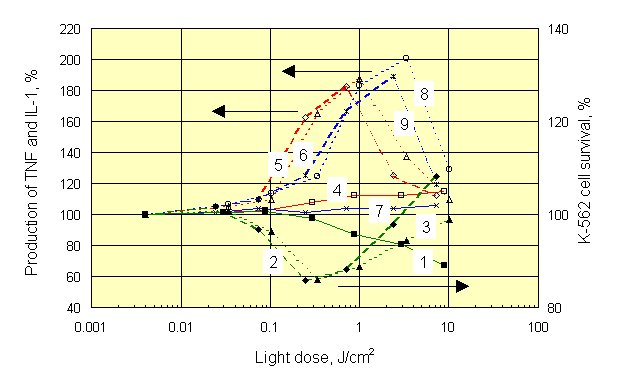
As we have noticed above, optimum conditions for photosensitized stimulation of immune reactions with PDT procedure are not yet determined. Therefore, we conducted experiments on mixed cell cultures which contained mouse splenocytes and target K-562 cells (20 : 1, respectively). Mixtures of various composition were studied: a) splenocytes plus K-562 separately dyed with Chlorine e6, Chlorine P6 or Photosan-3 (by incubation with dyes at room temperature for 24 hours), b) cells dyed plus cells not dyed, and c) cells irradiated plus cells non-irradiated in various combinations. Concentration of the dyes varied within a range 0,3 - 3,0 m g/ml.. Red (620-760 nm) and white (390-760 nm) light was used for irradiation of cultures at intensities of 15-35 mW/cm2. At the end of 24-h coculture, the survival of 3H-uridine prelabelled K-562 cells exposed to NK activity of splenocytes, as well as the concentrations of tumor necrosis factor (TNF) and interleukin-1 (IL-1) in supernatants were estimated. Typical results presented on Figure 4, were obtained with dyed and white light irradiated splenocytes which were cultured with dyed but non-irradiated K-562 target cells.
|
At all concentrations of dye, an apparent mirror symmetry was seen between photostimulated production of TNF and survival of K-562 targets. At the largest concentration of dye and at doses of light higher than 10 J/cm2, the decrease of photostimulatory effect, and even change of its sign was observed. We think it is a result of photochemical damage of splenocytes and loss by them of their functions. The similar results were obtained with the other dyes under white and red light, unessential quantitative differences were observed only. Role of light was investigated further using separate irradiation of K-562 target cultures and their mixed cultures with splenocytes (Figure 5).
 |
|
Despite different shape of dose-response curves after irradiation of different objects, mirror symmetry between production of TNF and K-562 cell survival was always observed. The maximum of TNF production for all dyes was associated with doses of light near 1 J/cm2, which corresponded to optimal dose of light needed for highest killing of K-562 target cells cocultured with splenocytes. At the same time, the maximum of IL-1 production was associated with those doses of light, which did not stimulate natural killer activity of splenocytes. It forces in a new fashion to look at a role IL-1 in the mechanism of anti-tumor immunity. Exactly the same results were obtained with Chlorines e6 and P6. Insignificant quantitative differences observed with the latter dyes can be seen by comparing appropriate curves on Figures 5 and 6.
It is necessary to notice, that by mixing irradiated dyed targets with non-dyed lymphocytes it was possible to receive more high enhancement of natural killer cell activity at light doses of 10-30 J/cm2. However, during irradiation of whole blood, i.e. in clinical conditions, such large doses of light could activate some other types of cell, bringing positive effect to nought. In particular, possible photochemical destruction of lymphocytes in irradiated mixed cell cultures was mentioned above (see Figures 5 and 6).
3.3. Evolutionary adaptation to light and mechanisms of action of light on living objects.
To understand obtained results as manifestation of evolutionary adaptation to natural light; we shall consider some fundamental photochemical properties of organic molecules. But at first we shall give the following statement. All molecules, of which living organism consists, can be divided into two groups. 1) The molecules, which do not practically absorb light in the field of radiation of the sun on a surface of ground. Also it is necessary to take into account optical and photochemical properties of surface layers of skin. These molecules can only play a role of substrates in any photosensitizes processes (examples - albumin, trypsin). 2) The molecules, capable to absorb light of the sun. As a consequence of evolutionary adaptation they have such properties, which provide animal with normal ability to live on light. Identification of these properties is an urgent problem of science. Almost everyone know, that haemoglobin transfers oxygen, providing breath, but few if any paid attention, that the same haemoglobin converts UV and almost the whole visible range of solar light into heat [4] with a factor of 106, thus transforming erythrocytes into traps for light and distributors of the heat over the organism, thereby reducing a thermal load of light on a body.
Using large number of the experimental facts as a basis [5], it is possible to approve, that organic molecules of the second group in lower excited states do not change a direction (character, sort) of their reactionary ability in comparison with their ground state. At absorption of a light quantum by a molecule, the rate constants of chemical reactions only increase. This statement can be justified theoretically. We shall consider reactions of photooxidation and photoreduction, using the one-electronic approach, as an example. At an absorption of a light quantum, the potential of ionization of an organic molecule decreases (increasing the ability of a molecule to give electron back) by the value of energy of excited state. Simultaneously, the affinity of a molecule to electron (i.e. the ability to capture electron) increases by the same value. Therefore, the direction of reactionary ability of a molecule (to take or to give electron) does not vary at photoenergization. It is regulated by properties of molecules of environment. Therefore, any light with low intensity (there are no two-quantum processes) does not introduce new biochemical responses to an organism, but only accelerates a part them, i.e. light accelerates biochemical responses, already existing in an organism. As a result, the instant balance of the network of biochemical processes varies; i.e. biochemical state of an organism varies.
Natural (solar, white) light, to which organism and its tissues are evolutionary adapted, accelerates such set of bioprocesses, which does not disturb total balance of biochemical responses, since the protective responses of an organism immediately (or with delay) eliminate abnormalities. As a result, the biochemical state of an organism remains practically unchanged. It constitutes, on our sight, photochemical aspect of adaptation of an organism to natural light. Light different from natural (down to monochromatic), to which living organisms had not been adapted during evolution, accelerates rather narrow set of biochemical responses, which disrupt natural balance of biochemical processes. In a common case, the protective responses of an organism are not sufficient because they can not resist to monochromatic light. Therefore, monochromatic light induces significant changes in biochemical state of organism. To our opinion, this reason makes it impossible to predict a priori either positive, or negative clinical effects of phototherapy (laserotherapy). We have observed this in the experiments with laser light [1, 2].
Another situation takes place in organism under the action of photosensitizers (photochemotherapy). Usually the sensitized photoresponses are new for an organized universe of normally proceeding biochemical processes in organism. The organism is not adapted to sensitized photoprocesses. It is possible to expect strong response of an organism to sensitized photoprocesses. In a particular case, photosensitized biological reaction can coincide by the result (by its product) with biological reaction normally occuring in organism. If the velocity of photosensitized production of such product extends acceptable limits (the range of stability of a biochemical state of organism), the balance of responses will be disturbed significantly and the transition to a new state of organism will occur. For example, singlet oxygen, a powerful cytotoxic agent, is permanently generated in tissues under the action of natural light, while not influencing on a normal state of tissues. However, under the PDT procedure much more singlet oxygen is generated which leads to destruction and/or modification of cells, brings changes to a state of organism, and provides therapeutic effect.
Thus it is necessary to take into account a circumstance important by its consequences. In all known applications of photosensitizers for medical purposes, the concentration of photosensitizer in tissues is low and does not substantially change optical properties of tissues. Thus, for example, according to our data and reports of other authors [15], the administration of dye (in therapeutic dose) during PDT leads to an additional weakening of light in a tissue by no more than 3-7 %, (we investigated spectrophotometrically thick (1-3 mm) sections of tissues). Therefore, photosensitizer is not an internal filter and does not significantly change light absorption of tissues. It allows us to make two important conclusions. (1) Under the use of photosensitizer in combination with monochromatic light (or other light, different from natural), the same photoresponses occur in a tissue as in the absence of photosensitizer, and they have the same efficiency. In other words, the combination of monochromatic light with photosensitizer will always exert multiple effect on a tissue and/or an organism (photochemistry due to absorption of light by endogenous chromophores plus photosensitized responses). 2) Under the use of natural (white) light, due to adaptation of tissues to it, the unique process, disturbing the balance of biochemical responses, is only the photosensitized process.
References
1 A.A.Akimov, A.S.Barchuk, M.L.Gel’fond, V.G.Maslov, N.B.Mihailova, R.P.Ogurtsov, T.P.Prokof’eva, V.V.Ryl’kov, I.E.Samsonova, A.N. Stukov and V.A.Filov, "New effects in photodynamic therapy of tumors", Proc. International Conference " Laser Optics’93 ", St. Petersburg, 1993, State Optical Institute, St. Petersburg, (1993) p. 621; Proc. SPIE, Vol. 2097, "Laser Applications", Arthur A. Mak; Ed, p.5-9, (1994).
2 V.V.Ryl’kov, V.G.Maslov, R.P.Ogurtsov, T.P.Prokof’eva and I.E.Samsonova, "Photostimulation of the organism protective reactions during photodynamic therapy of neoplasms", Proc. International Conference " New Achievements in Laser Medicine ", St. Petersburg, 1993, Institute for Laser Medicine, Moscow - St. Petersburg, p. 214-215 (1993).
3 T.I.Karu, "Photobiology of Low Power Laser Therapy", Hardwood Academic, London (1989).
4 M.Yu.Taras’ev and V.V.Ryl’kov, "Photochemistry of haemoglobin under native conditions", Biochemistry-Engl Tr., 56, 905-911 (1991).
5 A.N.Terenin, "Photonics of dye molecules", Nauka, Leningrad (1967).
6 B.C.Wilson, M.S.Patterson and D.M.Burns, "Effect of photosensitizers concentration in tissue on the penetration depth of photoactivating light", Lasers in Med. Sci., 1, 235-244 (1986).