| Papers and Posters | Site Home Page |
Photodynamic effect of deuteroporphyrin IX derivatives on
isolated nerve cell
A.B.Uzdensky1, A.V.Ivanov2,
A.V.Reshetnikov3, G.V.Ponomarev3,
O.Yu.Dergacheva1, A.A.Zhavoronkova1,
1)Rostov State University, Department of
Biophysics, Rostov-on-Don, 344090, Russia. uzd@krinc.ru
2)N.N. Blokhin Oncological Scientific Center, Russian Academy
of Medical Sciences,20, Kashirskoye shosse, Moscow, 115478,
Russia
3)Institute of Biomedical Chemistry, Russian Academy of Medical
Sciences,10, Pogodinskaya str., 119832, Moscow, Russia
![]()
ABSTRACT
Deuteroporphyrin IX derivatives are prospective PDT porphyrin photosensitizers (PS). The photodynamic effects of 6 new amphiphilic deuteroporphyrin derivatives with different hydrophobicity, as well as effects of known photosensitizers Photoheme and Photosens (used for comparison) on the firing of isolated crayfish mechanoreceptor neuron have been studied. After 30 min photosensitization, neurons were irradiated with He-Ne laser (632,8 nm, 0,3 W/cm2), and changes in neuron firing frequency were recorded. It has been shown that neuron firing is very sensitive to photodynamic impact and can serve as a sensitive indicator of cell photodamage. The comparison of dependencies of neuron lifetime on photosensitizer concentrations has provided comparison of their photodynamic efficiencies. The studied deuteroporphyrin IX derivatives have been found to be very potent PS. They induced irreversible firing abolition at pikomolar concentrations while Photoheme and Photosens were effective in the nanomolar range. The most effective PS were 4-(1-methyl-3-hydroxybutyl)- and 4-(1-methyl-2-acetyl-3-oxobutyl)-deuteroporphyrins. High photodynamic efficiencies of deuteroporphyrin derivatives were related to a weak dependence of photodynamic effect on sensitizer concentration, indicating that an initiation of several (3-5) chains of secondary processes such as free radical membrane damage by absorption of photon by photosensitizer molecule could take place. The main photosensitizer feature determining its intracellular localization and photodynamic efficiency has been amphiphilicity.
Keywords: photodynamic therapy, photodynamic effect, photosensitizers, deuteroporphyrin IX, porphyrin, Photosens, Photoheme, neuron, firing
![]()
1. INTRODUCTION
Photodynamic therapy (PDT) includes selective accumulation of photosensitizing substances (PS) in tumor and subsequent photogeneration of singlet oxygen 1O2 and other cytotoxic products causing malignant tissue destruction upon illumination1,2. Different compounds - porphyrins, chlorins, phthalocyanines, etc. are under examination in the search of the optimal PS1,3,4. Intracellular localization of PS which depends on its hydrophobicity and amphiphilicity is very important for its anti-cancer efficiency. An attractive feature of deuteroporphyrin IX derivatives (DP) is their high lipophilicity and, hence, the ability to photosensitize cellular membrane systems. In order to study the possible role of the position and character of side substituents endowing DP molecules with polarity or lipophilicity in their PD efficacy, we synthesized a number of 4-monosubstituted or 2,4-disubstituted DPs and studied their PD effect on the model systems5,6. The study is in progress now for 2-monosubstituted porphyrins as well as for 2-devinylchlorin e6 derivatives.
Recently isolated crayfish mechanoreceptor neuron has been proposed as a sensitive model for comparison of PSs and investigation of some mechanisms of PD effect at the cellular level7,8 . The structure, biochemical, electrophysiological, and photobiological features of this classic neurophysiological object are well studied9-12. This neuron is able to fire with a nearly constant rate during several hours, and at this stable background one can continuously record the dynamics of cell response to external impact from initial threshold shifts to terminal events leading to the cell death. In the present paper we have studied the dynamics of neuron responses to different 4-monosubstituted or 2,4-disubstituted DPs in order to compare their PD efficiencies.
![]()
2. METHODS
Slowly adapting muscle receptor organs of the crayfish Astacus leptodactilus were isolated as described by Wiersma et al.13. These were placed into a plexiglass chamber with van Harreveld saline (mM: NaCl - 205; KCL -5.4; NaHCO3 - 0.24; MgCl2 - 5.4; CaCl2 - 13.5; pH 7.2-7.4). In this preparation, stretch receptor neurons were capable of regular firing at a nearly constant rate for up to 8-12 hours. Neuron spikes were derived extracellularly from axons by the glass pipette suction electrodes, amplified (amplifier UU-90, Institute of Experimental Medicine, St.Petersbourg, Russia), with their frequency being converted into voltage by analog frequency meter (MFU-1, Institute of Experimental Medicine, St.Petersbourg, Russia) and continuously recorded by the chart-recorder (N-390, ZIP, Krasnodar, Russia). To test the irreversibility of neuron activity abolition we recorded neuron potentials 30-60 min after cessation of spikes and then additionally stimulated SRN by receptor muscle extension. The absence of spikes indicated that neuron had lost the ability to fire.
The experimental protocol was as follows: at the beginning of each experiment the initial neuron frequency level was set near 10-15 Hz by application of the appropriate receptor muscle extension. After 30 min 'control' recording of spike generation, the PS solution was added into the chamber. After the next 30 min, cells were irradiated with helium-neon laser (632,8 nm, 0.3 W/cm2, LGN-111, 'Polyaron', L'vov, Republic of Belarus) until the irreversible firing cessation. The irradiation power was measured by laser dosimeter (IMO-2N, 'Etalon', Volgograd, Russia). The irradiation exposures were as long as the neuron lifetimes.
The following photosensitizers were studied:
| 4-(1-methyl-2-acetyl-3-oxobutyl)deuteroporphyrin IX (4AcAc); | |
| 2,4-di(1-methyl-2-acetyl-3-oxobutyl) deuteroporphyrin IX (2,4diAcAc); | |
| 4-(1-methyl-3-oxobutyl)deuteroporphyrin IX (4Ac); | |
| 2,4-di(1-methyl-3-oxobutyl)deuteroporphyrin IX) (2,4Ac); | |
| 4-(1-methyl-3-hydroxybutyl)deuteroporphyrin IX (4OHbu) | |
| 2,4-di(1-methyl-3-hydroxybutyl)deuteroporphyrin IX (2,4diOHbu). |
The PD effects of these DPs were compared with the studied earlier14 PD effects of known photosensitizers:
| Photosens (PhS), sulphonated aluminum phthalocyanine, AlPcSn (synthesized at State research center NIOPIK, Moscow, Russia, in a group headed by Prof. G.N.Vorozhtsov). | |
| Photoheme (PhH), hematoporphyrin derivative (synthesized in Prof. A.F.Mironov's group, M.V. Lomonosov State Academy of Fine Chemical Technology, Moscow, Russia). |
The chemical formulae of the studied DPs are shown in Fig. 1, and their main physical and chemical characteristics are presented in Table 1. Light absorption spectra of these PSs were recorded by spectrophotometer Hitachi 557 (Japan), using 0.01 M borate buffer solution (pH 9.18) as a solvent. Chromatografic mobility indexes Rf characterizing lipophilicity of methyl esters of these compounds were determined by thin layer chromatography on Kieselgel 60 F254 plates (Merck) using mixture chloroform-ethanol-acetone (99:1:10). Amphiphilicity of these PS was estimated on the basis of the partition coefficient (Kp) in the system 1-octanol/phosphate buffer (pH 7.4).
![]()
Table 1. The main physical and chemical
features of deuteroporphyrin IX derivatives
Substance (PS) |
M.w. |
Visible absorption spectra, lambdamax ,nm (e*10-3) |
Amphiphilicity, Kp |
Lipophilicity, Rf |
2,4diOHbu |
1070 |
393 (135.18), 501 (9.66), 537 (7.75), 555 (5.96), 605 (3.10) |
17.00.5 |
0.05 |
4OHbu |
984 |
394 (191.12), 501 (10.62), 536 (8.52), 558 (6.58), 607 (2.99) |
28.00.2 |
0.27 |
2,4diAcAc |
1150 |
394 (177.44), 500 (12.67), 536 (10.08), 555 (7.92), 605 (3.60) |
19.40.9 |
0.45 |
2,4diAc |
1066 |
393 (128.80), 501 (8.64), 537 (6.77), 556 (52.62), 605 (22.55) |
10.00.5 |
0.48 |
4AcAc |
1024 |
394 (956.92), 501 (53.16), 536 (41.08), 560 (31.41), 605 (13.29) |
28.02.0 |
0.54 |
4Ac |
982 |
394 (180.58), 500 (10.62), 535 (7.92), 554 (6.76), 605 (3.67) |
9.10.1 |
0.56 |
PhH |
complex mixture; c.a.1260 |
396 ( |
1.50.1 |
- |
PhS |
complex mixture; c.a.1050 |
603 ( |
0.050.01 |
0 |
![]()
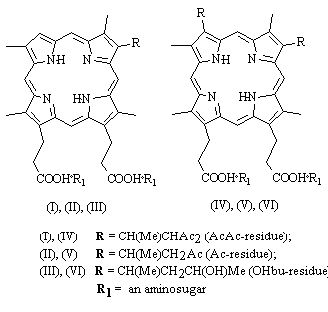
Fig. 1. Chemical formulae of deuteroporphyrin IX derivatives.
![]()
All experiments were accomplished without special thermostating at room temperature of 303
oC. Standard statistical methods including correlation and regression analysis14
were used. To compare efficiencies of different photosensitizers, we studied
concentration dependencies of neuron lifetime(T). Functions T(C) were
approximated by the power functions: T(C) = a*Cb, which are linear in
double logarithmic coordinates: lg T= lg a + b*l g C. Parameters a, and b
were determined by the method of least squares.
![]()
3. RESULTS
3.1. Hydrophobicity and amphiphilicity of deuteroporphyrin IX derivatives
According to Table 1 2,4diOHbu appeared to be the most hydrophilic DP photosensitizer. Its Rf=0.05 was, however, higher than for Photosens. Other DPs were markedly more lipophilic (Rf=0.27-0.56). The least amphiphilic PS among them was Photosens (Kp=0.05), Photoheme was more amphiphilic (Kp=1.5). All DPs were much more amphiphilic and could be subdivided into three groups: 4Ac and 2,4diAc (Kp=9.1 and 10.0), then 2,4diOHbu and 2,4diAcAc (Kp=17.0 and 19.4, respectively), and 4AcAc and 4OHbu were the most amphiphilic PSs (Kp=28.0).
3.2. Dynamics of neuron response to photodynamic effect of deuteroporphyrin IX derivatives
The unstained SRN was found to be insensitive tothe He-Ne laser irradiation lasting several hours nor to the addition of PS in the dark. However, they were very sensitive to the combination of these factors, i.e. to the PD effect. Neuron response dynamics included phases of firing acceleration or inhibition. Prolonged irradiation caused irreversible firing cessation that was considered to be the functional sign of the cell death. The following two main ways of firing abolition were observed: (a) firing acceleration followed by its abrupt abolition, or (b) gradual firing inhibition resulting in the irreversible cessation of spike generation. In both cases firing did not resume neither spontaneously, nor under additional adequate stimulation (receptor muscle extension). The dynamics of the cell response to PD impact (the alternating of firing excitation (E) and inhibition (I) phases) depended on PS type and concentration (Fig.2, Table 2).
![]()
Table 2. The
main types of neuron response dynamics to PD effect
| Responses | Firing changes |
| E | activation followed by abrupt firing cessation |
| EIE | activation, inhibition, and new activation followed by abrupt firing cessation |
| IE | activation and then acceleration followed by abrupt firing cessation |
| I | gradual inhibition until irreversible firing cessation |
| EI | activation and gradual inhibition until irreversible firing cessation |
| IEI | inhibition, activation, and new gradual inhibition until irreversible firing cessation |
![]()
As Table 3 shows, the initial response phase was acceleration of firing (E-, EI-, or EIE-responses) in 70-100 % neurons at the relatively high PS concentrations (> 10-7-10-6 M). More prominent difference between the neuron responses to various PSs was observed at the lesser concentrations ( < 10-7-10-9 M). In this case the neuron sensitization with 4AcAc, 4Ac, 4OHbu, or Photosens caused initial firing acceleration in 60-70 % cells. The sensitization with 2,4diOHbu or 2,4diAc caused, on the contrary, the initial inhibition of firing in 60-70 % neurons. 2,4diAcAc and Photoheme induced firing acceleration and inhibition in approximately one half of experiments.
Considering the terminal phases of the neuron response to PD effect (Table 3) one can subdivide all studied PSs into the following groups: (i) 71-75 % responses to sensitization with 4AcAc, 4Ac and Photosens were excitatory (E or EIE types) and culminated with abrupt firing abolition at both high and low PS concentrations. (ii) About 65 % responses to sensitization with 4OHbu were of I, EI, or IEI types with the irreversible firing cessation after prolonged gradual inhibition phase. This was observed both at high and at low PS concentrations. (iii) Excitatory final phases were dominant at high PS concentrations while inhibitory ones - at low concentrations of 2,4diOHbu; 2,4diAcAc; 2,4diAc or Photoheme.
![]()
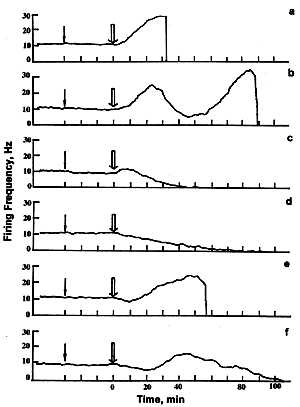
Fig. 2. The main types of neuron responses to photodynamic effect. A - E-response; B - EIE-response; C - EI-response; D - I-response; E - IE-response; F - IEI- response. Ordinate - firing frequency, Hz; abscissa - time, min.
![]()
3.3. Concentration dependencies
In order to compare PD efficiencies of different PSs we studied dependencies of neuron lifetimes T on PS concentrations C. These were approximated by the power functions: T(C) = a*Cb linear in the double logarithmic coordinates: lg T= lg a + b*lg C (Fig. 3). Parameters a and b determined by the least squares method are presented in the Table 3. The most effective PS occupy the left lower corner in the Fig. 3. These data show that PD efficiency is increased in the following series:
Photoheme 2,4diOHbu < Photosens <
2,4diAcAc< 2,4di Ac 4Ac < 4OHbu < 4AcAc
![]()
Table 3. The main types of
neuron responses to photodynamic effect of different photosensitizers
Photosensitizer |
Concentration, M |
Number of experiments |
The type of neuron response |
|||||
E |
EI |
EIE |
I |
IE |
IEI |
|||
| 2,4diOHbu | 10-8-10-5 |
23 |
21.7 |
17.4 |
21.7 |
17.4 |
0.0 |
21.7 |
10-8-5 10-7 |
12 |
0 |
8.3 |
25.0 |
33.3 |
0.0 |
33.3 |
|
10-6-10-5 |
11 |
45.4 |
27.3 |
18.2 |
0.0 |
0.0 |
9.1 |
|
| 4└˝└˝ | 10-12-10-5 |
30 |
40.0 |
10.0 |
26.6 |
6.7 |
6.7 |
10.0 |
10-12-10-9 |
19 |
21.0 |
15.8 |
26.3 |
10.5 |
10.5 |
15.8 |
|
5 10-9-10-5 |
11 |
77.8 |
0.0 |
27.2 |
0.0 |
0.0 |
0.0 |
|
| 4└˝ | 10-10-10-5 |
24 |
29.2 |
12.5 |
37.5 |
8.3 |
8.3 |
4.2 |
10-10-10-7 |
14 |
7.1 |
14.2 |
43.2 |
14.2 |
14.2 |
7.1 |
|
5 10-7-10-5 |
10 |
60.0 |
10.0 |
30.0 |
0 |
0 |
0 |
|
| 4╬═bu | 5 10-13-10-5 |
31 |
12.9 |
51.6 |
9.7 |
3.2 |
12.9 |
9.7 |
5 10-13-10-7 |
22 |
4.6 |
45.4 |
13.6 |
4.6 |
18.2 |
13.6 |
|
5 10-7-10-5 |
9 |
33.3 |
66.7 |
0 |
0 |
0 |
0 |
|
| 2,4di└˝└˝ | 5 10-10-10-5 |
21 |
22.8 |
19.1 |
22.8 |
14.3 |
0 |
21.0 |
5 10-10-10-7 |
13 |
7.7 |
30.8 |
15.4 |
15.4 |
0 |
30.8 |
|
5 10-7-10-5 |
8 |
50.0 |
0 |
37.5 |
12.5 |
0 |
0 |
|
| 2,4di└˝ | 10-11-10-5 |
25 |
20.0 |
12.0 |
20.0 |
32.0 |
4.0 |
12.0 |
10-11-5 10-8 |
15 |
6.7 |
20.0 |
13.3 |
46.7 |
0 |
13.3 |
|
10-7-10-5 |
10 |
40.0 |
0 |
30.0 |
10.0 |
10.0 |
10.0 |
|
| PhH | 5 10-9-2 10-5 |
16 |
25.0 |
13.0 |
31.0 |
25.0 |
0 |
6.0 |
5 10-9-2 10-7 |
9 |
11.1 |
11.1 |
22.2 |
44.4 |
0 |
11.1 |
|
5 10-7-2 10-5 |
7 |
42.9 |
14.2 |
42.9 |
0 |
0 |
0 |
|
| PhS | 10-9-10-5 |
23 |
7 |
21 |
64 |
4 |
0 |
4 |
10-9-2 10-7 |
13 |
0 |
32 |
58 |
5 |
0 |
5 |
|
6 10-7-10-5 |
10 |
22 |
0 |
78 |
0 |
0 |
0 |
|
![]()
Table 4. Statistical parameters
characterizing the dependence of neuron lifetime on photosensitizer concentrations
| Photosensitizer | Concentration range, M |
Number of experiments |
Correlation coefficient |
Regrassion
coefficients |
Student's
|
Fischer's
|
|
| 2,4di╬═bu | 10-8-10-5 |
23 |
-0.723 |
0.84 |
-0.46 |
4.80 |
23.0 |
| 4└˝└˝ | 10-12-10-5 |
30 |
-0.798 |
0.17 |
-0.27 |
7.01 |
49.2 |
| 4└˝ | 5 10-11-10-5 |
26 |
-0.756 |
0.72 |
-0.25 |
5.67 |
32.1 |
| 4╬═bu | 5 10-13-10-5 |
31 |
-0.822 |
0.54 |
-0.22 |
7.77 |
60.4 |
| 2,4di└˝└˝ | 5 10-10-10-5 |
21 |
-0.711 |
0.66 |
-0.30 |
4.40 |
19.4 |
| 2,4di└˝ | 10-11-10-5 |
26 |
-0.659 |
0.77 |
-0.22 |
4.29 |
18.4 |
| PhH | 5 10-9-2 10-5 |
16 |
-0.851 |
0.91 |
-0.51 |
6.07 |
36.8 |
| PhS | 10-9-10-5 |
27 |
-0.636 |
0.83 |
-0.33 |
4.13 |
17.0 |
![]()
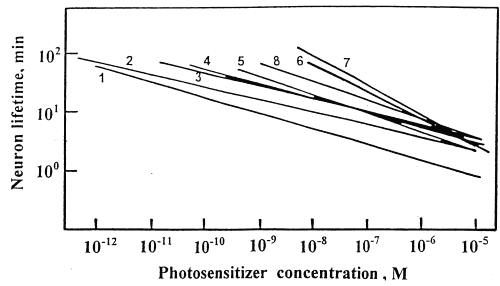
Fig.3. Neuron lifetime T (min) versus
concentrations C (M) of different photosensitizers:
(1) - 4AcAc; (2) - 4OHbu; (3) -2,4diAc; (4) - 4Ac; (5) - 2,4diAcAc; (6) - 2,4diOHbu; (7) -
PhH; (8) -PhS.
![]()
All DPs except 2,4diOHbu were much more efficient than Photoheme and Photosens and caused the cell death at pikomolar concentrations. All studied PSs could be divided onto the following groups: (I) 4AcAc and 4OHbu effective at C > 10-12 M; (II) 2,4diAc; 4Ac, and 2,4diAcAc effective at C > 10-11 M; (III) Photosens effective at C > 10-9 M, and (IV) 2,4-diOHbu and Photoheme effective at C > 10-8 M (Fig.3). One can also note that all 4-monosubstituted DPs were more efficient than the 2,4-disubstituted ones, and AcAc-derivatives were more efficient than Ac-derivatives. The most effective PSs were 4OHbu and 4AcAc.
A weak dependence of PD effect on PS concentration i.e. the low value of b is of importance for the cell killing capability of PS at low concentrations. In our case b=0.2-0.3 for all DPs except 2,4diOHbu. Therefore, one PS molecule absorbing the light quantum could induce 3-5 secondary cell lesions resulting in firing changes, perhaps, due to initiation of 3-5 chains of free radical damage of cellular membranes.
![]()
4. DISCUSSION
What reasons determine the neuron response dynamics to PD effect? It is known that singlet oxygen is a primary cytotoxic product of PD effect. During its lifetime of 10 nsec its diffusion path is only about 10 nm16. Therefore, it damages cellular structure in the nearest vicinity of PS localization. The latter depends on different factors, in particular on PS lipophilicity and amphiphilicity. In the course of the cell staining (sensitization) PS molecules first are adsorbed onto the plasma membrane, and them penetrate into the cell. Hydrophilic PSs with low amphiphilicity will enter the cell due to pinocytosis and end up in vesicles, endosomes and lysosomes17,18. Irradiation can disturb the plasma membrane integrity, and PSs will enter the cytosol and non-specifically sensitize different cellular structures19. Hydrophobic PSs with low amphiphilicity are dissolved in the lipid matrix of the plasma membrane and are difficult to go into the cytosol. In this case irradiation of the cell is not to change the PS localization. The most effective amphiphilic PSs have polar and non-polar groups in different parts of the molecule6,20. These are water-soluble compounds but meeting with the plasma membrane they pass into its lipid matrix, then diffuse into cytosol and can be included into the membranes of intracellular organelles such as endoplasmic reticulum, mitochondria, Golgi, etc.
DPs studied in the present work (except 2,4diOHbu) were much more effective than Photoheme, Photosens, and chlorins e6 and p6 (Table 5)21. Perhaps, it is connected with their higher amphiphilicity and lipophilicity and hence the higher cell-penetration ability. For example, very high PD efficiency of 4OHbu was presumably the result of its high amphiphilicity, but not of its moderate lipophilicity. 2,4OHbu with the moderate amphiphilicity was the least effective due to the least lipophilicity. The least amphiphilic and lipophilic Photosens and Photoheme were the least effective PSs. The distribution of PSs by amphiphilicity better correlated with the distribution by PD efficiency than the distribution by lipophilicity (Table 5). Therefore, PS amphiphilicity is more essential for predicting photosensitization effect than chromatographic retention coarsely approximating lipophilicity. Aside from these features, PD efficiency depends evidently on other PS characteristics such as ability to form hydrogen bonds, spectral characteristics, extinction, quantum yields of 1O2 generation, etc.
As assumed earlier 14,21, PD-induced firing acceleration was the result of free radical-induced plasma membrane damage and firing inhibition was mediated by the photoinjury of Ca2+-storing organelles such as mitochondria and endoplasmic reticulum and the consecutive Ca2+ release. Our data (Table 3,5) show that excitatory firing changes both in the initial and terminal neuron response phases were characteristic for photosensitization with PhS and 4-monosubstituted DP: 4AcAc and 4Ac. Two latter DPs were the most lipophilic and their photosensitization was presumably caused by the PS molecules dissolved in the lipid bilayer of the plasma membrane. On the contrary, PhS is hydrophilic and very weakly amphiphilic. Its effect was, possibly, for the most part mediated by the molecules around the plasma membrane. However, the significant inhibitory component of the neuron response to PhS-induced PD effect (the high percentage of EIE responses with a prominent inhibitory phase) indicates to PD-damage of Ca2+-storing organelles such as endoplasmic reticulum, mitochondria, etc. occurring due to PhS penetration into the cell upon irradiation19,22 . On the other hand, the inhibitory responses in both initial and terminal phases (that were presumably caused by PD injury of mitochondria and/or endoplasmic reticulum) were dominant at the photosensitization with 2,4-disubstituted DP. These PSs were of moderate amphiphilicity and different lipophilicity. Therefore, these properties were not the critical factors in the mechanism of neuron damage induced by these DPs. Perhaps, other reasons were more important for the neuron inactivation.
![]()
Table 5. Distribution of the studied
photosensitizers by photodynamic efficiency and physical-chemical properties
| Property | Photosensitizer distribution |
| Amphiphilicity | PhS <<PhH <<4└˝~2,4di└˝
< 2,4di╬═bu =2,4di└˝└˝ <4└˝└˝
~ 4╬═bu (0.05) (1.5) (9) (10) (17) (19) (28) (28) |
| Lipophilicity | PhS << 2,4di╬═bu <
4╬═bu < 2,4di└˝└˝~ 2,4di└˝ < 4└˝└˝~
4└˝ (0) (0.05) (0.27) (0.45) (0.48) (0.54) (0.56) |
| Neuron lifetime | PhH ~2.4di╬═bu < PhS < 2,4di└˝└˝ < 4└˝ 2,4di└˝ < 4╬═bu < 4└˝└˝ |
| The percentage of excitatory initial phases in neuron responses (E+EI+EIE) at low PS concentrations | 2,4di╬═bu < 2,4di└˝ < PhH
< 2,4di└˝└˝ < 4└˝└˝ ~4╬═bu = 4└˝ < PhS (33) (40) (44) (54) (63) (64) (64) (90) |
| The percentage of excitatory final phases in neuron responses (E+IE+EIE) at low PS concentrations | 2,4di└˝ ~2,4di└˝└˝
~2,4di╬═bu< PhH~4╬═bu
< 4└˝└˝ = PhS < 4└˝
(20) (23) (25) (33) (36) (58) (58) (65) |
The numerical values are given in the brackets.
![]()
5. CONCLUSIONS
| The firing of isolated crayfish neuron is very sensitive to PD impact and can serve as a sensitive indicator of cell photodamage. |
| The dependencies of neuron lifetime on PS concentrations allow to compare PD efficiencies of different photosensitizers. |
| The studied deuteroporphyrin IX derivatives were very potent PSs. They induced irreversible firing abolition at pikomolar concentrations while Photoheme and Photosens used in PD therapy were effective in the nanomolar range. |
| The most effective PSs were 4OHbu and 4AcAc. |
| High PD efficiencies of the DPs was related to the weak dependence of PD effect on PS concentration that is presumably due to initiation of several (3-5) chains of secondary processes such as free radical membrane damage upon the absorption of single photon by a PS molecule. |
| The main PS feature determining its intracellular localization and PD efficiency is amphiphilicity. |
![]()
6. ACKNOWLEDGEMENT
The work was supported by RFBR (grant 97-04-48092) and Competition Center for Fundamental Sciences at Saint-Petersbourg University (grant 095-0-10.0-31).
![]()
7. REFERENCES
1.B.W Henderson and T.J. Dougherty, "How does photodynamic therapy work?", Photochem. and Photobiol. 55, 145-157 (1992).
2. M. Ochsner, "Photophysical and photobiological processes in the photodynamic therapy of tumours", J. Photochem.Photobiol. B. Biol. 39, 1-18 (1997).
3.M. Korbelik, "Photosensitizers in photodynamic therapy", Period. Biol. 93, 563-574, 1991.
4.G. Jori, "Tumour photosensitizers: approaches to enhance the selectivity and efficience of photodynamic therapy", J. Photochem. Photobiol. B. Biol. 36, 87-93, 1996.
5. A.V.Ivanov, A.V. Reshetnickov, A.A. Dmitriev, A.T. Gradyushko, V.I. Shvetz, and G.V. Ponomarev, "Structural-Functional dependencies for some porphyrin photosensitizers", 2-nd All-Russian Photobiol. Meeting. Proceedings, Puschino, pp. 362-364, 1998. (in Russian)
6.A.V. Reshetnickov, V.I. Shvetz, and G.V. Ponomarev, "Water-soluble tetrapyrrolic photosensitizers for the photodynamic therapy of cancer (Review)", Progress in porphyrin chemistry. Vol. 2, Saint-Petersbourg Univ. Press, 1999 (in press) (in Russian).
7.A.B. Uzdensky, "Bioelectric changes in single neuron under photodynamic effect: comparison of different photosensitizers", IEEE JSTQE. 2, 984-988 (1996).
8.A.B. Uzdensky, O.Yu. Kutko, and N.V. Pasikova, "Single crayfish neuron as a new test-object for search and examination of PDT photosensitizers", Proc. SPIE, 2625, 512-518 (1996).
9.A.B. Kogan, V.F. Mashansky, G.M. Fedorenko, S.L. and Zaguskin, "Ultrastructure of crayfish mechanoreceptor neuron in the resting, rhythmic activity, and inhibition states induced by the adequate extension", Cytology, 16, 150-157 (1974) (in Russian).
9. E.E. Giacobini, "Chemical Studies of Individual Neurons. Neurosciences Research. II. Invertebrate nerve cel"l. New York : Acad. Press., pp. 111-202 (1969).
10. G.N. Akoev and N.P. Alekseev, "Functional organization of mechanoreceptors", Nauka, Leningrad, 1985. (in Russian).
11. A.B. Uzdensky, "Laser microirradiation of single nerve cell", Proc. SPIE, 1882, 254-267 (1993).
12. C.A.G Wiersma, E. Furshpan, and E. Florey, "Physiological and pharmacological observations on muscle organ of the crayfish, Cambarus clarkii Girard", J. Exp. Biol. 30, 136-151 (1953).
14. A. B. Uzdensky, "Photodynamic nerve cell killing: dynamics of electrophysiological responses and photosensitizers comparison" Proc. SPIE, 3191, 130-139 (1997).
15. B.M.Vladimirsky, Mathematical Methods in biology, Rostov Univ. Press, Rostov-on-Don, 1983. (in Russian)
16. J. Moan and K. Berg , "The photodegradation of porphyrins in cells can be used to estimate the lifetime of singlet oxygen", Photochem. Photobiol. 53, 549-553 (1991).
17.K Berg and J. Moan, "Lysosomes and microtubules as targets for photochemotherapy of cancer" Photochem Photobiol. 65, 403-409 (1997).
18. H. Schneckenburger, R. Sailer, M.H. Gschwend et al., "Uptake, subcellular localization , and phototoxicity of photosensitizing porphyrins" Proc. SPIE 2625, 96-104, 1996.
19. A.D. Scully, R.B. Ostler, A.J. MacRobert, A.W.Parker, C. de Lara, P. O'Neill and D. Phillips, "Laser line-scanning confocal fluorescence imaging of the photodynamic action of aluminum and zinc phthalocyanines in V79-4 Chinese hamster fibroblasts" Photochem. Photobiol. 68, 199-204 (1998).
20. J. Moan, Q. Peng, J.F. Evensen, K. Berg, A. Western and C. Rimington, "Photosensitizing efficiencies, tumor- and cellular uptake of different photosensitizing drugs relevant for photodynamic therapy of cancer" Photochem. Photobiol. 46, 713-721 (1987).
21. A.B. Uzdensky, A.A. Zhavoronkova and O.Y. Dergacheva, "The photodynamic effect of Photosens on single nerve cell: chemical modification", 7th Biennial congress of International Photodynamic Association" 7-9 July 1998. Nantes. France. CD-ROM Proceeding. P45 (1998).
22. A. Hubmert, A.Hermann, K. Uberriegler and B. Krammer, "Role of calcium in
photodynamically induced cell damage of human fibroblasts", Photochem. Photobiol.
64, 211-215 (1996).