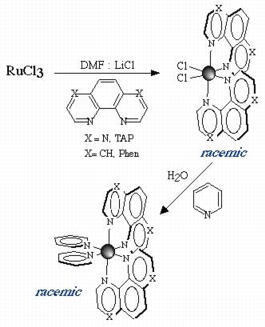
 Scheme : synthesis of [Ru(L)2(pyridine)2]2+ in two
steps
Scheme : synthesis of [Ru(L)2(pyridine)2]2+ in two
stepsSynthesis and Resolution of "Chiral Building Blocks"
 Scheme : synthesis of [Ru(L)2(pyridine)2]2+ in two
steps
Scheme : synthesis of [Ru(L)2(pyridine)2]2+ in two
steps
The choice of these precursor is based on the work published by the group of von Zelewsky et. al [2] who have showed that [Ru(phen)2(pyridine)2]2+ is particularly convinient as chiral building block as :
A limited number of other examples exist in the litterature as the [Ru(BPY)2(CO)2]2+ developed by Keene and coworkers [3] and [Ru(BPY)2(DMSO)Cl]1+ by Y. Inoue [4].
[1] B. Bosnich, F. Dwyer, Aust. J. Chem., 1966, 19, 2229
[2] X. Hua, A. von Zelewsky, Inorg. Chem., 1995, 34, 5791
[3] R. Keene, Coord. Chem. Rev., 1997, 166, 121
[4] D. Hesek, Y. Inoue, S. Everitt, H. Ishida, M. Kunieda, M. Drew, Chem. Commun., 1999, 403-404
Resolution of "Chiral Building Blocks"
I. [Ru(TAP)2(pyridine)2]2+
Scheme : Attemps of optical resolution of [Ru(TAP)2(pyridine)2]2+ with 3 different chiral counter-ions
In the case of [Ru(TAP)2(pyridine)2]2+, we first have tried the method of optical resolution described for [Ru(phen)2(pyridine)2]2+ by von Zelewsky and coworkers :
- Addition of the (L)-(+)-arsenyltartrate sodium salt to an aqueous solution of the complex does not lead to precipitation of any of the enantiomers of the complex. In the case of BPY and phen analogues however, it was shown that this resolving agent allows the specific precipitation of the L enantiomer [1].
As this method did not succeed we tried two different resolving agents :
- Addition of the (R)-(-)-camphorsulfonic sodium salt to an aqueous solution of the complex does not lead either to precipitation of any of the enantiomers of the complex. Camphorsulfonic acid salt was chosen as it has been applied succesfully for the resolution of Co complexes [2].
- The third resolving agent used, Trisphat, was tested at the University of Geneva by Pr. J. Lacour laboratory. This new optically active anion is particularly interesting for resolution of Ru(II) complexes as it presents also an octahedral structure which allows chiral discrimination among molecular propellers.
- Diastereoisomeric crystallisation was not succesfull either with the Trisphat anion.
- Ion pair chromatic resolution on SiO2 Thin Layer support did not yield to the separation of the enantiomers either althought this methods has been applied succesfully to [Ru(BPY)3]2+ [3].
- Enantioselective Extraction by the chiral lipophilic Trisphat anion did not succeed either. This method has been recently developed by J. Lacour and is schematically depicted on the figure above [4].
In order to achieve the optical resolution of this precursor, we will need to find out conditions of resolution excluding aqueous solutions. Indeed, all the method tested until know requires precipitation or extraction from an aqueous phase whereas the presence of 2 TAP ligands (rich in nitrogen) strongly modifies the polarity (increase) compared to phen ligand and thereby the solubility of this complex.
[1] X. Hua, A. von Zelewsky, Inorg. Chem., 1995, 34, 5791
[2] R. Marusak, M. Ivanca, K. Haller, G. Lappin, Inorg.Chem., 1991, 30, 618-623
[3] Lacour, J.; Torche-Haldimann, S.; Jodry, J. J.; Ginglinger, C.; Favarger, F.; Chem.Comun.,1998, 1733-1734
[4] J. Lacour results to be published
II. [Ru(phen)2(pyridine)2]2+
as described in X. Hua, A. von Zelewsky, Inorg. Chem., 1995, 34, 5791
Detremination of the Enantiomeric ExcessSpecific Optical Rotation and Circular Dichroïsm
Scheme : Optical Dispersion and Circular Dichroïsm of the enantiomers of [Ru(phen)2(pyridine)2]2+
The enantiomeric excess obtained following the resolution of [Ru(phen)2(pyridine)2]2+ with (L)-(+)-arsenyltartrate sodium salt was determined by the measurment of the optical rotation of this complex in aqueous solution and compared to the value determined in the litterature [1].
- for L-(+) [Ru(phen)2(pyridine)2]2+ : [a]D = + 479°.
From this value, by comparison with the litterature data, an enantiomeric excess (e.e.) of 96% is calculated. The assignement of the absolute configaration is done according to the litterature where application of the exciton theory on the circular dichroïsm spectrum has been performed. Von Zelewsky and coworkers used a different method to confirm the optical purity of the enantiomers of [Ru(phen)2(pyridine)2]2+ as the determination of optical rotation is only valuable with a reference of the optically pure complex which was then not available. In order to check the optical purity, they synthetised [Ru(phen)2(R,R-dach)]2+ (R,R-dach = (R,R)-1,2,-diaminocyclohexane) from each enatiomers they obtained and from the racemic mixture. Comparison of the 1H NMR spectrum of the diastereoisomers formed allowed them to set a limiting value of the e.e. and in the same time to evidence that the substitution of the two pyridine ligands by this enatiomerically pure ligand occurs with complete retention of the absolute configuration [1].
- The circular dichroïsm of the 2 enantiomers obtained is shown above. This spectrum is in agreement with litterature data and allows the assignement of the absolute configuration. The spectra corresponding to the 2 enatiomers are mirror images as expected but the relative intensities of the bands are slightly different indicating different enatiomeric excess for the 2 enantiomers.
Therefore, as the e.e. is sufficient, this precursor will be used in the "stereocontrolled" synthesis of optically pure Ru(II) complexes.