| Papers and Posters | Site Home Page |
CELL CYCLE DEPENDENT ACCUMULATION IN MITOSIS AFTER PHOTODYNAMIC THERAPY
Department of Biophysics, Institute for Cancer Research, The Norwegian Radium Hospital, Montebello, N-0310 Oslo 3 (Norway),
ABSTRACT
Human cervix carcinoma cells of the line NHIK 3025 were exposed to light after 18 h incubation with Photofrin. Before exposure to light the cells were synchronized by means of a reciprocal shaker. After photodynamic treatment the cell growth, mitotic index and cells survival were measured. The growth of NHIK 3025 cells treated with Photofrin for 18 h was delayed to a higher extent when the cells were treated with light in late S/early G2 and G2/M phases than when they were treated in G1 and early S phases. Only cells exposed to light 11 h (mid-S) and 14 h (late S/early G2) after synchronization accumulated in mitosis. The sensitivity of Photofrin treated and synchronized cells to photoinactivation correlated well with the accumulation of the cells in mitosis, except for cells exposed to light 18 h after synchronization. These cells, which were partly in G2M phase and partly in early G1 phase at the time of light exposure, were highly sensitive to photoinactivation, but accumulated only to a minor extent in mitosis.
INTRODUCTION
World-wide, photodynamic therapy (PDT) is being evaluated as a new and promising treatment modality of neoplastic diseases (1,2). The treatment is based on injection of photosensitizing dyes followed by exposure of the tumor area to high fluences of light at appropriate wavelengths. Porphyrins and structurally related compounds are applied for treatment of cancers, as well as for non tumoral diseases such as psoriasis (3), bacterial and viral eradication (4,5), vascular stenosis (6) and for tumour detection (7). Nevertheless, the application of PDT remains limited due to the limited penetration of light in tissues, the photosensitization of normal tissues and the remanent skin photosensitivity observed for several weeks to several months after treatment (8,9). The advantages of this method, as compared to other conventional cancer treatment modalities, are its low systemic toxicity and its ability to destroy tumors selectively (1,2).
It has previously been shown that NHIK 3025 cells accumulate in mitosis after PDT (10-13). Cells incubated for a short time (30 min) with Hematoporphyrin derivative (HpD) and treated with light in late stages of interphase accumulated in mitosis to a higher extent than cells exposed to light in G1 phase (10). Irradiation of cells after short-term incubation with porphyrins results in relatively more plasma membrane damage than irradiation after long-term incubation (14,15). A comparison between light treatment after short- and long term incubation might therefore give insight into the mechanisms for inhibition of cell division. In the present work we have studied accumulation of synchronized NHIK 3025 cells in mitosis after long-term incubation (18 h) with Photofrin and treatment with light.
MATERIALS AND METHODS
Cell cultivation. The established cell line NHIK 3025, derived from a carcinoma in situ of the cervix (
16) was used. The cells were subcultured twice a week in Medium E2a (17) containing 20 % human serum and 10 % horse serum. In order to load the cells with sufficient amounts of Photofrin they have in general been incubated in 3% serum (2% human serum and 1% horse serum) (13). Cell growth in 3% serum was found to be reduced (from a doubling time of 18 h to 24-30 h immediately after transfer to 3% serum) and complicates cell cycle studies. However, it was found that for cells grown continuously in 3% serum the doubling time was initially high, but was reduced with time of incubation and after 14 days of growth in 3% serum it was equal to cells grown in 30% serum. Thus, in all the experiments described in the present study the cells were grown in 3% serum for 2-3 weeks before treatment.Chemicals. Photofrin was provided by Photofrin Medical Inc. (Raritan,NJ).
Labelling with Photofrin, synchronization and irradiation. Cells (2 x 106) were seeded out in 175 cm2 plastic tissue-culture flasks (Nunclon) and incubated overnight before treatment. The cells were incubated for 18 h with 6
mg/ml Photofrin in E2a medium containing 3% serum. During incubation with Photofrin the cells were synchronized by isolation of mitotic cells as previously described (11). The mitotic and loosely attached cells were shaken off the substratum by a reciprocal shaker applied for 60 s. The medium was discarded and new E2a with 6 mg/ml Photofrin was added. One hour later the treatment with the reciprocal shaker was repeated and mitotic cells were pelleted, counted and seeded out in 25 cm2 plastic tissue-culture flasks (Nunclon) at a cell density of 50-100 x 103 cells/flask in E2a medium with 3% serum. The tissue culture flasks were flushed with 5% CO2 before being closed. The cells were exposed to light from a bank of 4 fluorescent tubes (mod. 3026,Appl. Photophysics, London) emitting light mainly around 405 nm. The fluence rate of the light reaching the cells was 15 W/m2. Immediately after illumination the medium was changed to E2a medium with 3 % serum and without Photofrin. The hole procedure was performed in a room kept at 370C.Cell proliferation. The multiplication of the cells was studied as previously described (
13) by counting in an inverted phase-contrast microscope (Leitz-Diavert) inside small areas marked on the flasks. Approximately 100 cells were counted for each area immediately after exposure to light. To avoid unwanted light exposure the light of the microscope was filtered through a flask with at least 100 m g Photofrin/ml E2a with 30 % serum. Photofrin bound to serum proteins has essentially the same absorption spectrum as Photofrin in cells. Cells counted every hour after incubation had the same proliferating rate and MI as similar cells counted only every 6th hour. This indicates that the filtered microscope light has no influence on the proliferation. The intensity of the filtered light at the position of the cells was always lower than 0.08 W/m2. All manipulations were performed in a room kept at 370C.Cell survival. Cell survival was studied by measuring the colony-forming ability of the cells as previously described (
13). One thousand cells per flask were inoculated in 25 cm2 plastic tissue-culture flasks (Nunclon) and treated as described above. After light exposure the cells were incubated for 8 days at 370C, and then fixed, stained and counted.RESULTS
NHIK 3025 cells were exposed to light at different time points after synchronization and cell growth measured as described in Materials and Methods (Fig.1). Cells treated with Photofrin showed a doubling of about 18 h as found for cells not treated with Photofrin (Fig.1, data not shown), while the growth of cells treated with Photofrin and light was in all cases delayed. The delay of cell growth was dependent on the time between synchronization and light exposure, i.e. on the phase of the cell cycle where the light exposure took place. Generally, the delay increased with increasing time between synchronization and light exposure.
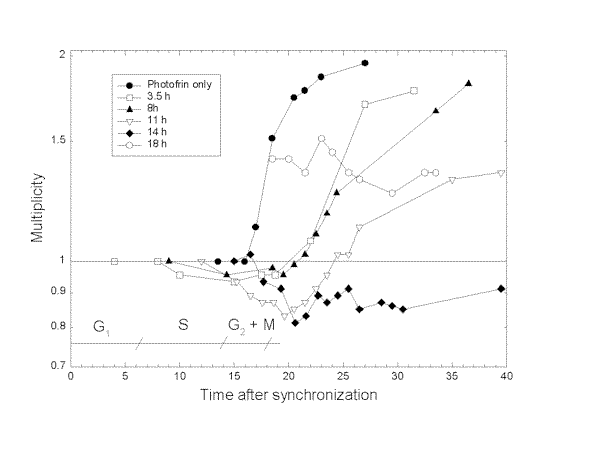
Fig.1. Multiplication of NHIK 3025 cells after treatment with Photofrin and light.
The cells were treated with 6 mg/ml Photofrin for 18 h and synchronized by mitotic shake off, exposed to 10 s of light at the time indicated on the figure and multiplication of the cells measured as described in Materials and Methods. Phases in the cell cycle are indicated on the figure.
It has previously been shown that NHIK 3025 cells accumulate in mitosis after PDT (10-13). Some of these mitotic cells die due to reduced or no formation of a mitotic spindle. This is probably caused by damage to tubulin (18). The mitotic index of the synchronized cells was measured continuously (Fig.2). Although the doubling time of cells exposed to PDT was in all cases increased by 7 hours or more their entry into mitosis was delayed by about 2-3 hours in all cases. The degree of accumulation in mitosis after PDT increased with time between synchronization and light exposure up to 14 hours after synchronization. Cells exposed to light 18 h after synchronization behaved similar to control cells (Figs. 2 and 3a). In accordance with the accumulation in mitosis after PDT the sensitivity of the cells to photoinactivation increased with time between synchronization and light exposure except for cells exposed to light 18 h after synchronization (Fig.3b). These cells were equally or slightly less sensitive to photoinactivation as compared to cells exposed to light 14 h after synchronization.
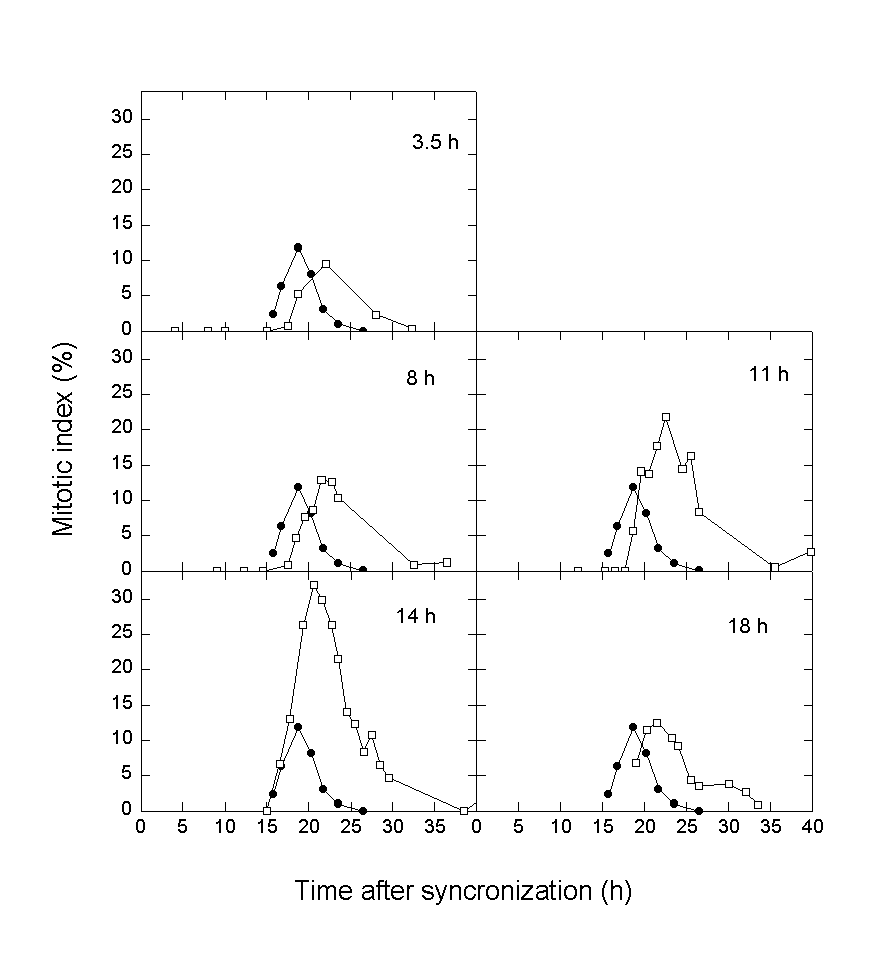
Fig.2. Accumulation of NHIK 3025 cells in mitosis after treatment with Photofrin and light.
The cells were treated with with 6 mg/ml Photofrin for 18 h and synchronized by mitotic shake off, exposed to 10 s of light at the time indicated on the figures and mitotic indices measured.
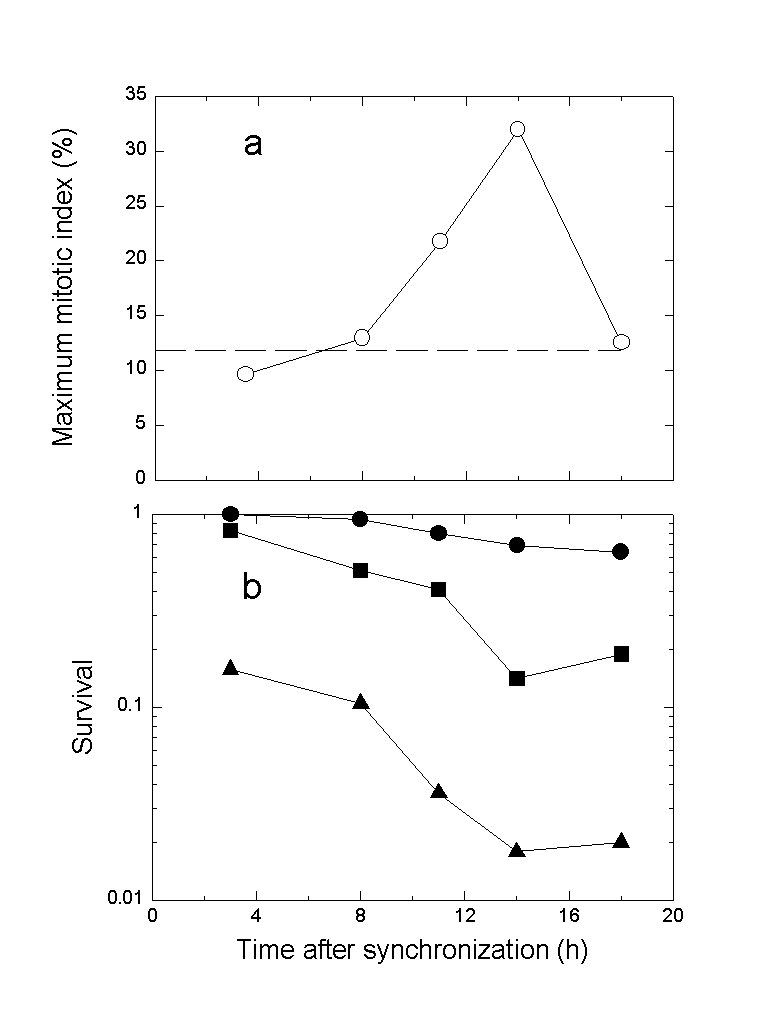
Fig.3. Maximum mitotic index (a) and cell survival (b) of synchronized cells treated with Photofrin and light.
a) Maximum mitotic index induced by PDT is described as a function of time after synchronization for light exposure. The results are based on Fig.2. b) Cell survival is measured as described in Materials and Methods. The cells were exposed to 10 s (#), 15 s (!) and 20 s (%) of light.
CONCLUSIONS
- The growth of NHIK 3025 cells treated with Photofrin for 18 h was more delayed when the cells were exposed to light in late S/early G2 and G2/M phases than when they were treated in G1 and early S phases.
- Only cells exposed to light 11 h (mid-S) and 14 h (late S/early G2) after synchronization accumulated in mitosis to a higher extent than cells not exposed to light.
- The photosensitivity of synchronized cells treated with Photofrin correlated well with the accumulation of the cells in mitosis except for cells exposed to light 18 h after synchronization. These cells, which were in G2M phase or in early G1 phase at the time of light exposure, were highly sensitive to photoinactivation, but accumulated only to a minor extent in mitosis.
REFERENCES
1. Henderson, B. and Dougherty, T.J. How does photodynamic therapy work? Photochem. Photobiol. 55: 145-157, 1992.
2. Moan, J. and Berg, K. Photochemotherapy of cancer: experimental research. Photochem. Photobiol., 55: 931-948, 1992.
3. Berns, M.W., Rettenmaier, M., McCullough, J., Coffey, J., Wile, A., Berman, M., DiSaia, P., and Weinstein, G. Response of psoriasis to red laser light (630 nm) following systemic injection of hematoporphyrin derivative. Lasers Surg Med, 4: 73-77, 1984.
4. Nir, U., Ladan, H., Malik, Z., and Nitzan, Y. In vivo effects of porphyrins on bacterial DNA. J. Photochem. Photobiol., B - Biology. 11: 295-306, 1991.
5. Rywkin, S., Ben-Hur, E., Malik, Z., Prince, A.M., Li, Y.S., Kenney, M.E., Oleinick, N.L., and Horowitz, B. New phthalocyanines for photodynamic virus inactivation in red blood cell concentrates. Photochem Photobiol, 60: 165-170, 1994.
6. Dartsch, P.C., Coppenrath, E., Coppenrath, K., and Ischinger, T. Photodynamic therapy of vascular stenosis: results from cell culture studies on human endothelial cells. Coron Artery Dis, 4: 207-213, 1993.
7. Andersson-Engels, S., Johansson, J., Svanberg, K., and Svanberg, S. Fluorescence imaging and point measurements of tissue: applications to the demarcation of malignant tumors and atherosclerotic lesions from normal tissue. Photochem Photobiol, 53: 807-814, 1991.
8. Wooten, R.S., Smith, K.C., Ahlquist, D.A., Muller, S.A., and Balm, R.K. Prospective study of cutaneous phototoxicity after systemic hematoporphyrin derivative. Lasers Surg Med, 8: 294-300, 1988.
9. Dougherty, T.J., Cooper, M.T., and Mang, T.S. Cutaneous phototoxic occurrences in patients receiving Photofrin. Lasers Surg Med, 10: 485-488, 1990.
10. Christensen, T. Multiplication of human NHIK 3025 cells exposed to porphyrins in combination with light. Br J Cancer, 44: 433-439, 1981.
11. Berg, K., Steen, H.B., Winkelman, J.W., and Moan, J. Synergistic effects of photoactivated tetra(4-sulfonatophenyl)porphine and nocodazole on microtubule assembly, accumulation of cells in mitosis and cell survival. J. Photochem. Photobiol., B - Biology. 13: 59-70, 1992.
12. Berg, K. and Moan, J. Mitotic inhibition by phenylporphines and tetrasulfonated aluminium phthalocyanine in combination with light. Photochem. Photobiol., 56: 333-339, 1992.
13. Berg, K. and Moan, J. Photodynamic effects of Photofrin II on cell division in human NHIK 3025 cells. Int J Radiat Biol, 53: 797-811, 1988.
14. Kessel, D. and Kohn, K.I. Transport and binding of mesoporphyrin IX by leukemia L1210 cells. Cancer Res. 40: 303-307, 1980.
15. Moan, J., Christensen, T., and Jacobsen, P.B. Photodynamic effects on cells in vitro labeled with hematoporphyrin derivative. Photobiochem. Photobiophys. 7: 349-358, 1984.
16. Nordbye, K. and Oftebro, R. Establishment of four new cell strains from uterine cervix.I. Exp. Cell Res. 58: 4581969.
17. Puck, T.T., Cieciura, S.J., and Fisher, H. Clonal growth in vitro of human cell with fibroblastic morphology. J. Exp. Med. 106: 145-165, 1957.
18. Berg, K. The unpolymerized form of tubulin is the target for microtubule inhibition by photoactivated tetra(4-sulfonatophenyl)porphine. Biochim. Biophys. Acta, 1135: 147-153, 1992.